Distinct timing of neutrophil spreading and stiffening during phagocytosis
- PMID: 35318004
- PMCID: PMC9072703
- DOI: 10.1016/j.bpj.2022.03.021
Distinct timing of neutrophil spreading and stiffening during phagocytosis
Abstract
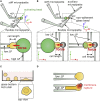
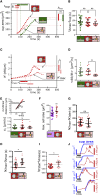
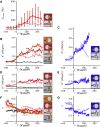
Phagocytic cells form the first line of defense in an organism, engulfing microbial pathogens. Phagocytosis involves cell mechanical changes that are not yet well understood. Understanding these mechanical modifications promises to shed light on the immune processes that trigger pathological complications. Previous studies showed that phagocytes undergo a sequence of spreading events around their target followed by an increase in cell tension. Seemingly in contradiction, other studies observed an increase in cell tension concomitant with membrane expansion. Even though phagocytes are viscoelastic, few studies have quantified viscous changes during phagocytosis. It is also unclear whether cell lines behave mechanically similarly to primary neutrophils. We addressed the question of simultaneous versus sequential spreading and mechanical changes during phagocytosis by using immunoglobulin-G-coated 8- and 20-μm-diameter beads as targets. We used a micropipette-based single-cell rheometer to monitor viscoelastic properties during phagocytosis by both neutrophil-like PLB cells and primary human neutrophils. We show that the faster expansion of PLB cells on larger beads is a geometrical effect reflecting a constant advancing speed of the phagocytic cup. Cells become stiffer on 20- than on 8-μm beads, and the relative timing of spreading and stiffening of PLB cells depends on target size: on larger beads, stiffening starts before maximal spreading area is reached but ends after reaching maximal area. On smaller beads, the stiffness begins to increase after cells have engulfed the bead. Similar to PLB cells, primary cells become stiffer on larger beads but start spreading and stiffen faster, and the stiffening begins before the end of spreading on both bead sizes. Our results show that mechanical changes in phagocytes are not a direct consequence of cell spreading and that models of phagocytosis should be amended to account for causes of cell stiffening other than membrane expansion.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Cell surface topography controls phagocytosis and cell spreading: The membrane reservoir in neutrophils.Biochim Biophys Acta Mol Cell Res. 2020 Dec;1867(12):118832. doi: 10.1016/j.bbamcr.2020.118832. Epub 2020 Aug 27. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32860836
-
Visualizing the Early Stages of Phagocytosis.J Vis Exp. 2017 Feb 3;(120):54646. doi: 10.3791/54646. J Vis Exp. 2017. PMID: 28191879 Free PMC article.
-
Mechanics of neutrophil phagocytosis: behavior of the cortical tension.J Cell Sci. 2005 May 1;118(Pt 9):1789-97. doi: 10.1242/jcs.02275. Epub 2005 Apr 12. J Cell Sci. 2005. PMID: 15827090
-
Phagocytosis--the mighty weapon of the silent warriors.Microsc Res Tech. 2002 Jun 15;57(6):421-31. doi: 10.1002/jemt.10096. Microsc Res Tech. 2002. PMID: 12112425 Review.
-
Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis.Int J Mol Sci. 2019 Mar 19;20(6):1383. doi: 10.3390/ijms20061383. Int J Mol Sci. 2019. PMID: 30893856 Free PMC article. Review.
Cited by
-
A theoretical model of efficient phagocytosis driven by curved membrane proteins and active cytoskeleton forces.Soft Matter. 2022 Dec 21;19(1):31-43. doi: 10.1039/d2sm01152b. Soft Matter. 2022. PMID: 36472164 Free PMC article.
-
Quantifying both viscoelasticity and surface tension: Why sharp tips overestimate cell stiffness.Biophys J. 2024 Jan 16;123(2):210-220. doi: 10.1016/j.bpj.2023.12.008. Epub 2023 Dec 12. Biophys J. 2024. PMID: 38087780 Free PMC article.
-
The aspect ratio effect on the cytotoxicity of inert nano-particles flips depending on particle thickness, and is one of the reasons for the literature inconsistency.Nanoscale Adv. 2022 Sep 29;4(24):5257-5269. doi: 10.1039/d2na00453d. eCollection 2022 Dec 6. Nanoscale Adv. 2022. PMID: 36540111 Free PMC article.
-
Rod-shaped Polymerized Salicylic Acid Particles Modulate Neutrophil Transendothelial Migration in Acute Inflammation.Adv Healthc Mater. 2025 Jun;14(16):e2404955. doi: 10.1002/adhm.202404955. Epub 2025 May 9. Adv Healthc Mater. 2025. PMID: 40348611 Free PMC article.
References
-
- Flannagan R.S., Jaumouillé V., Grinstein S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012;7:61–98. - PubMed
-
- Hallett M.B., editor. Molecular and Cellular Biology of Phagocytosis. Springer International Publishing; 2020.
-
- Niedergang F., Grinstein S. How to build a phagosome: new concepts for an old process. Curr. Opin. Cell Biol. 2018;50:57–63. - PubMed
-
- Ostrowski P.P., Grinstein S., Freeman S.A. Diffusion barriers, mechanical forces, and the biophysics of phagocytosis. Dev. Cell. 2016;38:135–146. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

