Histone deacetylase 5 is a phosphorylation substrate of protein kinase D in osteoclasts
- PMID: 35318161
- PMCID: PMC9035101
- DOI: 10.1016/j.bone.2022.116393
Histone deacetylase 5 is a phosphorylation substrate of protein kinase D in osteoclasts
Abstract
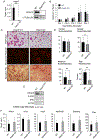
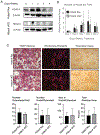
Protein kinase D (PRKD) family kinases are required for formation and function of osteoclasts. However, the substrates of PRKD in osteoclasts are unknown. To identify PRKD-dependent protein phosphorylation in osteoclasts, we performed a quantitative LC-MS/MS phosphoproteomics screen for proteins showing differential phosphorylation in osteoclasts after treatment with the PRKD inhibitor CRT0066101. We identified 757 phosphopeptides showing significant changes following PRKD inhibition. Among the changes, we found a group of 13 proteins showing decreased phosphorylation at PRKD consensus phosphorylation motifs. This group includes histone deacetylase 5 (HDAC5), which is a previously validated PRKD target. Considering this known interaction, work suggesting HDACs may be important regulators of osteoclasts, and studies suggesting potential functional redundancy between HDACs, we further investigated the relationship between PRKD and class IIa HDACs in osteoclasts. We confirmed that CRT0066101 inhibits phosphorylation of endogenous HDAC5 and to a lesser extent HDAC4, whereas HDAC7 phosphorylation was not affected. Osteoclast cultures from Hdac5 global knockout mice displayed impaired differentiation and reduced ability to resorb bone, while conditional knockout of Hdac4 in osteoclasts showed no phenotype in vitro or in vivo. The inhibitory effect of CRT0066101 was reduced in Hdac5 KO osteoclasts. Together these data indicate that the PRKD/HDAC5 axis contributes to osteoclast formation in vitro and suggest that this pathway may contribute to regulation of skeletal dynamics in vivo.
Keywords: Bone remodeling; Histone deacetylases; Osteoclast differentiation; Protein kinase D; Protein phosphorylation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Protein kinase D1 mediates class IIa histone deacetylase phosphorylation and nuclear extrusion in intestinal epithelial cells: role in mitogenic signaling.Am J Physiol Cell Physiol. 2014 May 15;306(10):C961-71. doi: 10.1152/ajpcell.00048.2014. Epub 2014 Mar 19. Am J Physiol Cell Physiol. 2014. PMID: 24647541 Free PMC article.
-
Converse role of class I and class IIa HDACs in the progression of atrial fibrillation.J Mol Cell Cardiol. 2018 Dec;125:39-49. doi: 10.1016/j.yjmcc.2018.09.010. Epub 2018 Oct 12. J Mol Cell Cardiol. 2018. PMID: 30321539
-
Cyclic AMP represses pathological MEF2 activation by myocyte-specific hypo-phosphorylation of HDAC5.J Mol Cell Cardiol. 2020 Aug;145:88-98. doi: 10.1016/j.yjmcc.2020.05.018. Epub 2020 May 30. J Mol Cell Cardiol. 2020. PMID: 32485181
-
Class IIa HDACs - new insights into their functions in physiology and pathology.FEBS J. 2015 May;282(9):1736-44. doi: 10.1111/febs.13061. Epub 2014 Oct 27. FEBS J. 2015. PMID: 25244360 Review.
-
Regulation of Osteoclast Differentiation and Skeletal Maintenance by Histone Deacetylases.Molecules. 2019 Apr 6;24(7):1355. doi: 10.3390/molecules24071355. Molecules. 2019. PMID: 30959867 Free PMC article. Review.
References
-
- Briggs AM, Cross MJ, Hoy DG, Sànchez-Riera L, Blyth FM, Woolf AD, March L, Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health, The Gerontologist 56(Suppl_2) (2016) S243–S255. - PubMed
-
- Brown JP, Antiresorptives: Safety Concerns-Clinical Perspective, Toxicol Pathol 45(7) (2017) 859–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

