Efficacy, safety and cost-effectiveness of methotrexate, adalimumab or their combination in non-infectious non-anterior uveitis: a protocol for a multicentre, randomised, parallel three arms, active-controlled, phase III open label with blinded outcome assessment study
- PMID: 35318229
- PMCID: PMC8943738
- DOI: 10.1136/bmjopen-2021-051378
Efficacy, safety and cost-effectiveness of methotrexate, adalimumab or their combination in non-infectious non-anterior uveitis: a protocol for a multicentre, randomised, parallel three arms, active-controlled, phase III open label with blinded outcome assessment study
Abstract
Introduction: Non-infectious uveitis include a heterogeneous group of sight-threatening and incapacitating conditions. Their correct management sometimes requires the use of immunosuppressive drugs (ISDs), prescribed in monotherapy or in combination. Several observational studies showed that the use of ISDs in combination could be more effective than and as safe as their use in monotherapy. However, a direct comparison between these two treatment strategies has not been carried out yet.
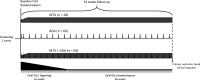
Methods and analysis: The Combination THerapy with mEthotrexate and adalImumAb for uveitis (CoTHEIA) study is a phase III, multicentre, prospective, randomised, single-blinded with masked outcome assessment, parallel three arms with 1:1:1 allocation, active-controlled, superiority study design, comparing the efficacy, safety and cost-effectiveness of methotrexate, adalimumab or their combination in non-infectious non-anterior uveitis. We aim to recruit 192 subjects. The duration of the treatment and follow-up will last up to 52 weeks, plus 70 days follow-up with no treatment. The complete and maintained resolution of the ocular inflammation will be assessed by masked evaluators (primary outcome). In addition to other secondary measurements of efficacy (quality of life, visual acuity and costs) and safety, we will identify subjects' subgroups with different treatment responses by developing prediction models based on machine learning techniques using genetic and proteomic biomarkers.
Ethics and dissemination: The protocol, annexes and informed consent forms were approved by the Reference Clinical Research Ethic Committee at the Hospital Clínico San Carlos (Madrid, Spain) and the Spanish Agency for Medicines and Health Products. We will elaborate a dissemination plan including production of materials adapted to several formats to communicate the clinical trial progress and findings to a broad group of stakeholders. The promoter will be the only access to the participant-level data, although it can be shared within the legal situation.
Trial registration number: 2020-000130-18; NCT04798755.
Keywords: clinical trials; ophthalmology; rheumatology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- WHO . The world health report, global data on visual impairments, 2010. Available: http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical