Presynaptic Interactions between Trigeminal and Cervical Nociceptive Afferents Supplying Upper Cervical Lamina I Neurons
- PMID: 35318285
- PMCID: PMC9053849
- DOI: 10.1523/JNEUROSCI.0025-22.2022
Presynaptic Interactions between Trigeminal and Cervical Nociceptive Afferents Supplying Upper Cervical Lamina I Neurons
Abstract
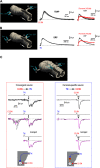
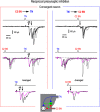
Cervical and trigeminal afferents innervate neighboring cranial territories, and their convergence on upper cervical dorsal horn neurons provides a potential substrate for pain referral in primary headache syndromes. Lamina I neurons are central to this mechanism, as they relay convergent nociceptive input to supraspinal pain centers. Unfortunately, little is known about the interactions between trigeminal and cervical afferents supplying Lamina I neurons. Here, we used rats of both sexes to show that cervical and trigeminal afferents interact via presynaptic inhibition, where monosynaptic inputs to Lamina I neurons undergo unidirectional as well as reciprocal presynaptic control. This means that afferent-driven presynaptic inhibition shapes the way trigeminal and cervical Aδ-fiber and C-fiber input reaches Lamina I projection neurons (PNs) and local-circuit neurons (LCNs). We propose that this inhibition provides a feedforward control of excitatory drive to Lamina I neurons that regulates their convergent and cervical-specific or trigeminal-specific processing modes. As a consequence, disruption of the trigeminal and cervical afferent-driven presynaptic inhibition may contribute to development of primary headache syndromes.SIGNIFICANCE STATEMENT Cervical and trigeminal afferents innervate neighboring cranial territories, and their convergence on upper cervical dorsal horn neurons provides a potential substrate for pain referral in primary headache syndromes. Lamina I neurons are central to this mechanism as they relay convergent nociceptive input to supraspinal pain centers. Here, we show that cervical and trigeminal afferents interact via presynaptic inhibition, where inputs to Lamina I neurons undergo unidirectional as well as reciprocal control. The afferent-driven presynaptic inhibition shapes the trigeminocervical Aδ-fiber and C-fiber input to Lamina I neurons. This inhibition provides control of excitatory drive to Lamina I neurons that regulates their convergent and cervical-specific or trigeminal-specific processing modes. Disruption of this control may contribute to development of primary headache syndromes.
Keywords: C2 spinal nerve; dorsal root potentials; nociceptive afferents; presynaptic inhibition; trigeminal nerve; trigeminocervical complex.
Copyright © 2022 the authors.
Figures




Similar articles
-
Processing of trigeminocervical nociceptive afferent input by neuronal circuity in the upper cervical lamina I.Pain. 2022 Feb 1;163(2):362-375. doi: 10.1097/j.pain.0000000000002342. Pain. 2022. PMID: 33990106
-
Trigeminal Aδ- and C-afferent supply of lamina I neurons in the trigeminocervical complex.Pain. 2019 Nov;160(11):2612-2623. doi: 10.1097/j.pain.0000000000001659. Pain. 2019. PMID: 31356449
-
Primary afferent-driven presynaptic inhibition of C-fiber inputs to spinal lamina I neurons.Prog Neurobiol. 2020 May;188:101786. doi: 10.1016/j.pneurobio.2020.101786. Epub 2020 Mar 12. Prog Neurobiol. 2020. PMID: 32173398
-
[Neurobiology of visceral pain].Schmerz. 2014 Jun;28(3):233-51. doi: 10.1007/s00482-014-1402-x. Schmerz. 2014. PMID: 24903037 Review. German.
-
Convergence of cervical and trigeminal sensory afferents.Curr Pain Headache Rep. 2003 Oct;7(5):377-83. doi: 10.1007/s11916-003-0037-x. Curr Pain Headache Rep. 2003. PMID: 12946291 Review.
Cited by
-
Contralateral Afferent Input to Lumbar Lamina I Neurons as a Neural Substrate for Mirror-Image Pain.J Neurosci. 2023 May 3;43(18):3245-3258. doi: 10.1523/JNEUROSCI.1897-22.2023. Epub 2023 Mar 22. J Neurosci. 2023. PMID: 36948583 Free PMC article.
-
Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study.J Clin Med. 2025 Feb 24;14(5):1510. doi: 10.3390/jcm14051510. J Clin Med. 2025. PMID: 40094996 Free PMC article.
References
-
- Alvarez-Leefmans FJ, Nani A, Marquez S (1998) Chloride transport, osmotic balance, and presynaptic inhibition. In: Presynaptic inhibition and neural control (Rudomin P, Romo R, Mendel LM, eds), pp 50–79. New York: Oxford University Press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
