Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors
- PMID: 35318315
- PMCID: PMC8940900
- DOI: 10.1038/s41467-022-29155-1
Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors
Abstract
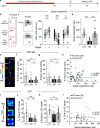
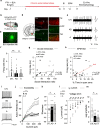
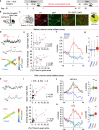
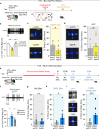
Anxiety disorders are complex diseases, and often co-occur with depression. It is as yet unclear if a common neural circuit controls anxiety-related behaviors in both anxiety-alone and comorbid conditions. Here, utilizing the chronic social defeat stress (CSDS) paradigm that induces singular or combined anxiety- and depressive-like phenotypes in mice, we show that a ventral tegmental area (VTA) dopamine circuit projecting to the basolateral amygdala (BLA) selectively controls anxiety- but not depression-like behaviors. Using circuit-dissecting ex vivo electrophysiology and in vivo fiber photometry approaches, we establish that expression of anxiety-like, but not depressive-like, phenotypes are negatively correlated with VTA → BLA dopamine neuron activity. Further, our optogenetic studies demonstrate a causal link between such neuronal activity and anxiety-like behaviors. Overall, these data establish a functional role for VTA → BLA dopamine neurons in bi-directionally controlling anxiety-related behaviors not only in anxiety-alone, but also in anxiety-depressive comorbid conditions in mice.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Kessler RC, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch. Gen. Psychiatry. 1994;51:8–19. - PubMed
-
- Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. - PubMed
-
- Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. - PubMed
-
- Tolu S, et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry. 2013;18:382–393. - PubMed
-
- King-Kallimanis B, Gum AM, Kohn R. Comorbidity of depressive and anxiety disorders for older Americans in the national comorbidity survey-replication. Am. J. Geriatr. Psychiatry. 2009;17:782–792. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

