Progranulin improves neural development via the PI3K/Akt/GSK-3β pathway in the cerebellum of a VPA-induced rat model of ASD
- PMID: 35318322
- PMCID: PMC8941112
- DOI: 10.1038/s41398-022-01875-4
Progranulin improves neural development via the PI3K/Akt/GSK-3β pathway in the cerebellum of a VPA-induced rat model of ASD
Abstract
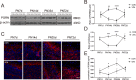
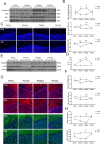
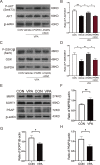
Autism spectrum disorder (ASD) is a neurodevelopmental disease featuring social interaction deficits and repetitive/stereotyped behaviours; the prevalence of this disorder has continuously increased. Progranulin (PGRN) is a neurotrophic factor that promotes neuronal survival and differentiation. However, there have not been sufficient studies investigating its effect in animal models of autism. This study investigated the effects of PGRN on autistic phenotypes in rats treated with valproic acid (VPA) and assessed the underlying molecular mechanisms. PGRN was significantly downregulated in the cerebellum at postnatal day 14 (PND14) and PND35 in VPA-exposed rats, which simultaneously showed defective social preference, increased repetitive behaviours, and uncoordinated movements. When human recombinant PGRN (r-PGRN) was injected into the cerebellum of newborn ASD model rats (PND10 and PND17), some of the behavioural defects were alleviated. r-PGRN supplementation also reduced cerebellar neuronal apoptosis and rescued synapse formation in ASD rats. Mechanistically, we confirmed that PGRN protects neurodevelopment via the PI3K/Akt/GSK-3β pathway in the cerebellum of a rat ASD model. Moreover, we found that prosaposin (PSAP) promoted the internalisation and neurotrophic activity of PGRN. These results experimentally demonstrate the therapeutic effects of PGRN on a rat model of ASD for the first time and provide a novel therapeutic strategy for autism.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Abnormal spatiotemporal expression pattern of progranulin and neurodevelopment impairment in VPA-induced ASD rat model.Neuropharmacology. 2021 Sep 15;196:108689. doi: 10.1016/j.neuropharm.2021.108689. Epub 2021 Jun 25. Neuropharmacology. 2021. PMID: 34175324
-
Mouse nerve growth factor suppresses neuronal apoptosis in valproic acid-induced autism spectrum disorder rats by regulating the phosphoinositide-3-kinase/serine/threonine kinase signaling pathway.Pharmacogenet Genomics. 2023 Jul 1;33(5):101-110. doi: 10.1097/FPC.0000000000000498. Epub 2023 May 2. Pharmacogenet Genomics. 2023. PMID: 37261937
-
Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3β.Protein Cell. 2010 Jun;1(6):552-62. doi: 10.1007/s13238-010-0067-1. Epub 2010 Jul 7. Protein Cell. 2010. PMID: 21204008 Free PMC article.
-
Vitamin A supplementation ameliorates prenatal valproic acid-induced autism-like behaviors in rats.Neurotoxicology. 2022 Jul;91:155-165. doi: 10.1016/j.neuro.2022.05.008. Epub 2022 May 17. Neurotoxicology. 2022. PMID: 35594946
-
Progranulin's Protective Mechanisms and Therapeutic Potential in Cardiovascular Disease.Cells. 2025 May 22;14(11):762. doi: 10.3390/cells14110762. Cells. 2025. PMID: 40497937 Free PMC article. Review.
Cited by
-
NEAT1 Promotes Valproic Acid-Induced Autism Spectrum Disorder by Recruiting YY1 to Regulate UBE3A Transcription.Mol Neurobiol. 2025 Jan;62(1):846-860. doi: 10.1007/s12035-024-04309-y. Epub 2024 Jun 26. Mol Neurobiol. 2025. PMID: 38922486
-
Building better brains: the pleiotropic function of neurotrophic factors in postnatal cerebellar development.Front Mol Neurosci. 2023 May 12;16:1181397. doi: 10.3389/fnmol.2023.1181397. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37251644 Free PMC article. Review.
-
Progranulin Protects against Hyperglycemia-Induced Neuronal Dysfunction through GSK3β Signaling.Cells. 2023 Jul 7;12(13):1803. doi: 10.3390/cells12131803. Cells. 2023. PMID: 37443837 Free PMC article.
-
Granulin loss of function in human mature brain organoids implicates astrocytes in TDP-43 pathology.Stem Cell Reports. 2023 Mar 14;18(3):706-719. doi: 10.1016/j.stemcr.2023.01.012. Epub 2023 Feb 23. Stem Cell Reports. 2023. PMID: 36827976 Free PMC article.
-
Preclinical Interventions in Mouse Models of Frontotemporal Dementia Due to Progranulin Mutations.Neurotherapeutics. 2023 Jan;20(1):140-153. doi: 10.1007/s13311-023-01348-6. Epub 2023 Feb 13. Neurotherapeutics. 2023. PMID: 36781744 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

