Brain-wide projection reconstruction of single functionally defined neurons
- PMID: 35318336
- PMCID: PMC8940919
- DOI: 10.1038/s41467-022-29229-0
Brain-wide projection reconstruction of single functionally defined neurons
Abstract
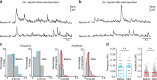
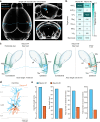
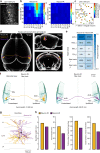
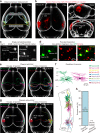
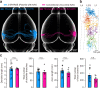
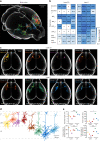
Reconstructing axonal projections of single neurons at the whole-brain level is currently a converging goal of the neuroscience community that is fundamental for understanding the logic of information flow in the brain. Thousands of single neurons from different brain regions have recently been morphologically reconstructed, but the corresponding physiological functional features of these reconstructed neurons are unclear. By combining two-photon Ca2+ imaging with targeted single-cell plasmid electroporation, we reconstruct the brain-wide morphologies of single neurons that are defined by a sound-evoked response map in the auditory cortices (AUDs) of awake mice. Long-range interhemispheric projections can be reliably labelled via co-injection with an adeno-associated virus, which enables enhanced expression of indicator protein in the targeted neurons. Here we show that this method avoids the randomness and ambiguity of conventional methods of neuronal morphological reconstruction, offering an avenue for developing a precise one-to-one map of neuronal projection patterns and physiological functional features.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mapping the Function of Whole-Brain Projection at the Single Neuron Level.Adv Sci (Weinh). 2022 Nov;9(33):e2202553. doi: 10.1002/advs.202202553. Epub 2022 Oct 13. Adv Sci (Weinh). 2022. PMID: 36228099 Free PMC article.
-
Reconstruction of 1,000 Projection Neurons Reveals New Cell Types and Organization of Long-Range Connectivity in the Mouse Brain.Cell. 2019 Sep 19;179(1):268-281.e13. doi: 10.1016/j.cell.2019.07.042. Epub 2019 Sep 5. Cell. 2019. PMID: 31495573 Free PMC article.
-
In Utero Electroporation Approaches to Study the Excitability of Neuronal Subpopulations and Single-cell Connectivity.J Vis Exp. 2017 Feb 15;(120):55139. doi: 10.3791/55139. J Vis Exp. 2017. PMID: 28287556 Free PMC article.
-
Single-neuron axonal reconstruction: The search for a wiring diagram of the brain.J Comp Neurol. 2019 Sep 1;527(13):2190-2199. doi: 10.1002/cne.24674. Epub 2019 Mar 21. J Comp Neurol. 2019. PMID: 30859571 Review.
-
Method for labeling and reconstruction of single neurons using Sindbis virus vectors.J Chem Neuroanat. 2019 Oct;100:101648. doi: 10.1016/j.jchemneu.2019.05.002. Epub 2019 Jun 8. J Chem Neuroanat. 2019. PMID: 31181303 Review.
Cited by
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases.Mol Neurodegener. 2023 Jul 20;18(1):49. doi: 10.1186/s13024-023-00634-3. Mol Neurodegener. 2023. PMID: 37475056 Free PMC article. Review.
-
Daily two-photon neuronal population imaging with targeted single-cell electrophysiology and subcellular imaging in auditory cortex of behaving mice.Front Cell Neurosci. 2023 Mar 3;17:1142267. doi: 10.3389/fncel.2023.1142267. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36937184 Free PMC article.
-
NMNAT2 supports vesicular glycolysis via NAD homeostasis to fuel fast axonal transport.Mol Neurodegener. 2024 Jan 29;19(1):13. doi: 10.1186/s13024-023-00690-9. Mol Neurodegener. 2024. PMID: 38282024 Free PMC article.
-
Mapping the Function of Whole-Brain Projection at the Single Neuron Level.Adv Sci (Weinh). 2022 Nov;9(33):e2202553. doi: 10.1002/advs.202202553. Epub 2022 Oct 13. Adv Sci (Weinh). 2022. PMID: 36228099 Free PMC article.
-
Brain-wide 3D neuron detection and mapping with deep learning.Neurophotonics. 2025 Apr;12(2):025012. doi: 10.1117/1.NPh.12.2.025012. Epub 2025 May 20. Neurophotonics. 2025. PMID: 40401216 Free PMC article.
References
-
- Pollak Dorocic I, et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

