Concentration-dependent dual effects of exogenous sucrose on nitrogen metabolism in Andrographis paniculata
- PMID: 35318399
- PMCID: PMC8940917
- DOI: 10.1038/s41598-022-08971-x
Concentration-dependent dual effects of exogenous sucrose on nitrogen metabolism in Andrographis paniculata
Abstract
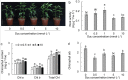
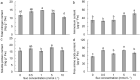
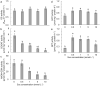
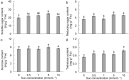
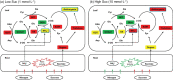
The effects of exogenous sucrose (Suc) concentrations (0, 0.5, 1, 5, 10 mmol L-1) on carbon (C) and nitrogen (N) metabolisms were investigated in a medicinal plant Andrographis paniculata (Chuanxinlian). Suc application with the concentration of 0.5-5 mmol L-1 significantly promoted plant growth. In contrast, 10 mmol L-1 Suc retarded plant growth and increased contents of anthocyanin and MDA and activity of SOD in comparison to 0.5-5 mmol L-1 Suc. Suc application increased contents of leaf soluble sugar, reducing sugar and trerhalose, as well as isocitrate dehydrogenase (ICDH) activity, increasing supply of C-skeleton for N assimilation. However, total leaf N was peaked at 1 mmol L-1 Suc, which was consistent with root activity, suggesting that exogenous Suc enhanced root N uptake. At 10 mmol L-1 Suc, total leaf N and activities of glutamine synthase (GS), glutamate synthase (GOGAT), NADH-dependent glutamate dehydrogenase (NADH-GDH) and glutamic-pyruvic transaminase (GPT) were strongly reduced but NH4+ concentration was significantly increased. The results revealed that exogenous Suc is an effective stimulant for A. paniculata plant growth. Low Suc concentration (e.g. 1 mmol L-1) increased supply of C-skeleton and promoted N uptake and assimilation in A. paniculata plant, whereas high Suc concentration (e.g. 10 mmol L-1) uncoupled C and N metabolisms, reduced N metabolism and induced plant senescence.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

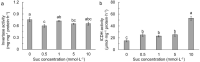
Similar articles
-
Dihydroporphyrin iron (III) enhances low temperature tolerance by increasing carbon and nitrogen metabolism in Andrographis paniculata.Front Plant Sci. 2025 Jan 3;15:1522481. doi: 10.3389/fpls.2024.1522481. eCollection 2024. Front Plant Sci. 2025. PMID: 39830946 Free PMC article.
-
Organic nitrogen sources promote andrographolide biosynthesis by reducing nitrogen metabolism and increasing carbon accumulation in Andrographis paniculata.Plant Physiol Biochem. 2021 Jul;164:82-91. doi: 10.1016/j.plaphy.2021.04.016. Epub 2021 May 5. Plant Physiol Biochem. 2021. PMID: 33975147
-
Exogenous γ-aminobutyric acid (GABA) alleviates nitrogen deficiency by mediating nitrate uptake and assimilation in Andrographis paniculata seedlings.Plant Physiol Biochem. 2023 May;198:107700. doi: 10.1016/j.plaphy.2023.107700. Epub 2023 Apr 13. Plant Physiol Biochem. 2023. PMID: 37086691
-
Role of sucrose in modulating the low-nitrogen-induced accumulation of phenolic compounds in lettuce (Lactuca sativa L.).J Sci Food Agric. 2020 Dec;100(15):5412-5421. doi: 10.1002/jsfa.10592. Epub 2020 Jul 23. J Sci Food Agric. 2020. PMID: 32562270
-
Photorespiration-dependent increases in phospho enolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-alpha-ketoglutarate aminotransferase.Planta. 2002 Apr;214(6):877-86. doi: 10.1007/s00425-001-0692-2. Epub 2001 Dec 14. Planta. 2002. PMID: 11941464
Cited by
-
Dihydroporphyrin iron (III) enhances low temperature tolerance by increasing carbon and nitrogen metabolism in Andrographis paniculata.Front Plant Sci. 2025 Jan 3;15:1522481. doi: 10.3389/fpls.2024.1522481. eCollection 2024. Front Plant Sci. 2025. PMID: 39830946 Free PMC article.
-
Application of root exudates derived from peanut/maize intercropping system promotes peanut growth and yield via modulating nitrogen turnover processes.BMC Plant Biol. 2025 Jul 29;25(1):977. doi: 10.1186/s12870-025-06994-w. BMC Plant Biol. 2025. PMID: 40731256 Free PMC article.
-
An ideal leaf spraying strategy of brown sugar for edible medicinal plants of Viola inconspicua.NPJ Sci Food. 2024 Nov 22;8(1):99. doi: 10.1038/s41538-024-00343-1. NPJ Sci Food. 2024. PMID: 39572563 Free PMC article.
References
-
- Ruan YL. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014;65:33–67. - PubMed
-
- Shahri W, Ahmad SS, Tahir I. Sugar signaling in plant growth and development. In: Hakeem K, Rehman R, Tahir I, editors. Plant Signaling: Understanding the Molecular Crosstalk. Springer; 2014. pp. 93–116.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

