VIVA1: a more invasive subclone of MDA-MB-134VI invasive lobular carcinoma cells with increased metastatic potential in xenograft models
- PMID: 35318435
- PMCID: PMC9276762
- DOI: 10.1038/s41416-022-01778-7
VIVA1: a more invasive subclone of MDA-MB-134VI invasive lobular carcinoma cells with increased metastatic potential in xenograft models
Abstract
Background: Invasive lobular carcinoma (ILC) is the second most common type of breast cancer. As few tools exist to study ILC metastasis, we isolated ILC cells with increased invasive properties to establish a spontaneously metastasising xenograft model.
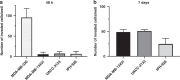
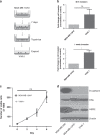
Methods: MDA-MB-134VI ILC cells were placed in transwells for 7 days. Migrated cells were isolated and expanded to create the VIVA1 cell line. VIVA1 cells were compared to parental MDA-MB-134VI cells in vitro for ILC marker expression and relative proliferative and invasive ability. An intraductally injected orthotopic xenograft model was used to assess primary and metastatic tumour growth in vivo.
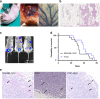
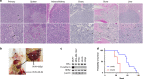
Results: Similar to MDA-MB-134VI, VIVA1 cells retained expression of oestrogen receptor (ER) and lacked expression of E-cadherin, however showed increased invasion in vitro. Following intraductal injection, VIVA1 and MDA-MB-134VI cells had similar primary tumour growth and survival kinetics. However, macrometastases were apparent in 7/10 VIVA1-injected animals. Cells from a primary orthotopic tumour (VIVA-LIG43) were isolated and showed similar proliferative rates but were also more invasive than parental cells. Upon re-injection intraductally, VIVA-LIG43 cells had more rapid tumour growth with similar metastatic incidence and location.
Conclusions: We generated a new orthotopic spontaneously metastasising xenograft model for ER+ ILC amenable for the study of ILC metastasis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response.Cancer Res. 2014 Mar 1;74(5):1463-74. doi: 10.1158/0008-5472.CAN-13-2779. Epub 2014 Jan 14. Cancer Res. 2014. PMID: 24425047 Free PMC article.
-
Mediator of DNA Damage Checkpoint 1 (MDC1) Is a Novel Estrogen Receptor Coregulator in Invasive Lobular Carcinoma of the Breast.Mol Cancer Res. 2021 Aug;19(8):1270-1282. doi: 10.1158/1541-7786.MCR-21-0025. Epub 2021 May 4. Mol Cancer Res. 2021. PMID: 33947745 Free PMC article.
-
Comprehensive Phenotypic Characterization of Human Invasive Lobular Carcinoma Cell Lines in 2D and 3D Cultures.Cancer Res. 2018 Nov 1;78(21):6209-6222. doi: 10.1158/0008-5472.CAN-18-1416. Epub 2018 Sep 18. Cancer Res. 2018. PMID: 30228172 Free PMC article.
-
Comprehensive Review of Molecular Mechanisms and Clinical Features of Invasive Lobular Cancer.Oncologist. 2021 Jun;26(6):e943-e953. doi: 10.1002/onco.13734. Epub 2021 Mar 16. Oncologist. 2021. PMID: 33641217 Free PMC article. Review.
-
Invasive lobular carcinoma: an understudied emergent subtype of breast cancer.Breast Cancer Res Treat. 2022 Jun;193(2):253-264. doi: 10.1007/s10549-022-06572-w. Epub 2022 Mar 26. Breast Cancer Res Treat. 2022. PMID: 35347549 Review.
References
-
- Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133:713–23. - PubMed
-
- Weigelt B, Geyer FC, Natrajan R, Lopez-Garcia MA, Ahmad AS, Savage K, et al. The molecular underpinning of lobular histological growth pattern: a genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J Pathol. 2010;220:45–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

