Porcine intraepithelial lymphocytes undergo migration and produce an antiviral response following intestinal virus infection
- PMID: 35318455
- PMCID: PMC8941121
- DOI: 10.1038/s42003-022-03205-2
Porcine intraepithelial lymphocytes undergo migration and produce an antiviral response following intestinal virus infection
Abstract
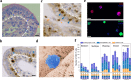
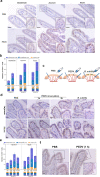
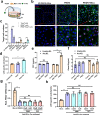
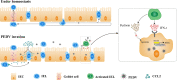
The location of intraepithelial lymphocytes (IELs) between epithelial cells provide a first line of immune defense against enteric infection. It is assumed that IELs migrate only along the basement membrane or into the lateral intercellular space (LIS) between epithelial cells. Here, we identify a unique transepithelial migration of porcine IELs as they move to the free surface of the intestinal epithelia. The major causative agent of neonatal diarrhea in piglets, porcine epidemic diarrhea virus (PEDV), increases the number of IELs entering the LIS and free surface of the intestinal epithelia, driven by chemokine CCL2 secreted from virus-infected intestinal epithelial cells. Remarkably, only virus pre-activated IELs inhibits PEDV infection and their antiviral activity depends on the further activation by virus-infected cells. Although high levels of perforin is detected in the co-culture system, the antiviral function of activated IELs is mainly mediated by IFN-γ secretion inducing robust antiviral response in virus-infected cells. Our results uncover a unique migratory behavior of porcine IELs as well as their protective role in the defense against intestinal infection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Intraepithelial lymphocytes in the pig intestine: T cell and innate lymphoid cell contributions to intestinal barrier immunity.Front Immunol. 2022 Dec 8;13:1048708. doi: 10.3389/fimmu.2022.1048708. eCollection 2022. Front Immunol. 2022. PMID: 36569897 Free PMC article. Review.
-
Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling.J Virol. 2018 Jan 30;92(4):e01677-17. doi: 10.1128/JVI.01677-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187542 Free PMC article.
-
Porcine Intestinal Enteroids: a New Model for Studying Enteric Coronavirus Porcine Epidemic Diarrhea Virus Infection and the Host Innate Response.J Virol. 2019 Feb 19;93(5):e01682-18. doi: 10.1128/JVI.01682-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541861 Free PMC article.
-
Quantitative Proteomic Analysis Reveals Antiviral and Anti-inflammatory Effects of Puerarin in Piglets Infected With Porcine Epidemic Diarrhea Virus.Front Immunol. 2020 Feb 26;11:169. doi: 10.3389/fimmu.2020.00169. eCollection 2020. Front Immunol. 2020. PMID: 32174911 Free PMC article.
-
Porcine epidemic diarrhea virus inhibits dsRNA-induced interferon-β production in porcine intestinal epithelial cells by blockade of the RIG-I-mediated pathway.Virol J. 2015 Aug 18;12:127. doi: 10.1186/s12985-015-0345-x. Virol J. 2015. PMID: 26283628 Free PMC article.
Cited by
-
Intraepithelial lymphocytes in the pig intestine: T cell and innate lymphoid cell contributions to intestinal barrier immunity.Front Immunol. 2022 Dec 8;13:1048708. doi: 10.3389/fimmu.2022.1048708. eCollection 2022. Front Immunol. 2022. PMID: 36569897 Free PMC article. Review.
-
Prolactin promotes transepithelial migration of lymphocytes through CCL2.Poult Sci. 2025 Aug;104(8):105322. doi: 10.1016/j.psj.2025.105322. Epub 2025 May 20. Poult Sci. 2025. PMID: 40446683 Free PMC article.
-
Transcriptomics yields valuable information regarding the response mechanisms of Chinese Min pigs infected with PEDV.Front Vet Sci. 2023 Dec 11;10:1295723. doi: 10.3389/fvets.2023.1295723. eCollection 2023. Front Vet Sci. 2023. PMID: 38192721 Free PMC article.
-
Development and function of natural TCR+ CD8αα+ intraepithelial lymphocytes.Front Immunol. 2022 Dec 7;13:1059042. doi: 10.3389/fimmu.2022.1059042. eCollection 2022. Front Immunol. 2022. PMID: 36569835 Free PMC article. Review.
-
Taking intestinal microbes and the immune system as opportunities to maintain the intestinal health of piglets: review.Anim Biosci. 2025 Sep;38(9):1827-1840. doi: 10.5713/ab.25.0100. Epub 2025 May 19. Anim Biosci. 2025. PMID: 40400191 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

