Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure
- PMID: 35318460
- PMCID: PMC9156408
- DOI: 10.1038/s41380-022-01513-x
Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure
Abstract
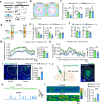
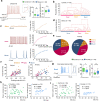
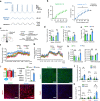
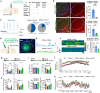
The central nervous system has evolved to coordinate the regulation of both the behavior response to the external environment and homeostasis of energy expenditure. Recent studies have indicated the dorsomedial ventromedial hypothalamus (dmVMH) as an important hub that regulates both innate behavior and energy homeostasis for coping stress. However, how dmVMH neurons control neuronal firing pattern to regulate chronic stress-induced anxiety and energy expenditure remains poorly understood. Here, we found enhanced neuronal activity in VMH after chronic stress, which is mainly induced by increased proportion of burst firing neurons. This enhancement of VMH burst firing is predominantly mediated by Cav3.1 expression. Optogenetically evoked burst firing of dmVMH neurons induced anxiety-like behavior, shifted the respiratory exchange ratio toward fat oxidation, and decreased food intake, while knockdown of Cav3.1 in the dmVMH had the opposite effects, suggested that Cav 3.1 as a crucial regulator. Interestingly, we found that fluoxetine (anxiolytics) could block the increase of Cav3.1 expression to inhibit the burst firing, and then rescued the anxiety-like behaviors and energy expenditure changes. Collectively, our study first revealed an important role of Cav3.1-driven bursting firing of dmVMH neurons in the control of anxiety-like behavior and energy expenditure, and provided potential therapeutic targets for treating the chronic stress-induced emotional malfunction and metabolism disorders.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Energy state alters regulation of proopiomelanocortin neurons by glutamatergic ventromedial hypothalamus neurons: pre- and postsynaptic mechanisms.J Neurophysiol. 2021 Mar 1;125(3):720-730. doi: 10.1152/jn.00359.2020. Epub 2021 Jan 13. J Neurophysiol. 2021. PMID: 33441043 Free PMC article.
-
Connexions between the dorsomedial division of the ventromedial hypothalamus and the dorsal periaqueductal grey matter are critical in the elaboration of hypothalamically mediated panic-like behaviour.Behav Brain Res. 2017 Feb 15;319:135-147. doi: 10.1016/j.bbr.2016.11.026. Epub 2016 Nov 14. Behav Brain Res. 2017. PMID: 27856260
-
Relevance of dorsomedial hypothalamus, dorsomedial division of the ventromedial hypothalamus and the dorsal periaqueductal gray matter in the organization of freezing or oriented and non-oriented escape emotional behaviors.Behav Brain Res. 2015 Oct 15;293:143-52. doi: 10.1016/j.bbr.2015.07.013. Epub 2015 Jul 20. Behav Brain Res. 2015. PMID: 26205826
-
Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism.Nutrition. 1997 May;13(5):403-11. doi: 10.1016/s0899-9007(97)91277-6. Nutrition. 1997. PMID: 9225331 Review.
-
Hypothalamic control of energy expenditure and thermogenesis.Exp Mol Med. 2022 Apr;54(4):358-369. doi: 10.1038/s12276-022-00741-z. Epub 2022 Mar 17. Exp Mol Med. 2022. PMID: 35301430 Free PMC article. Review.
Cited by
-
Ventromedial hypothalamus relays chronic stress inputs and exerts bidirectional regulation on anxiety state and related sympathetic activity.Front Cell Neurosci. 2023 Dec 14;17:1281919. doi: 10.3389/fncel.2023.1281919. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38161999 Free PMC article.
-
Increased sympathetic outflow induced by emotional stress aggravates myocardial ischemia-reperfusion injury via activation of TLR7/MyD88/IRF5 signaling pathway.Inflamm Res. 2023 May;72(5):901-913. doi: 10.1007/s00011-023-01708-0. Epub 2023 Mar 18. Inflamm Res. 2023. PMID: 36933018
-
Targeting the central and peripheral nervous system to regulate bone homeostasis: mechanisms and potential therapies.Mil Med Res. 2025 Mar 20;12(1):13. doi: 10.1186/s40779-025-00600-8. Mil Med Res. 2025. PMID: 40108680 Free PMC article. Review.
-
CaMKIIa Neurons of the Ventromedial Hypothalamus Mediate Wakefulness and Anxiety-like Behavior.Neurochem Res. 2023 Aug;48(8):2463-2475. doi: 10.1007/s11064-023-03925-9. Epub 2023 Apr 4. Neurochem Res. 2023. PMID: 37014492
-
Two distinct cell types of the medial mammillary body forming segregated subcircuits.Mol Psychiatry. 2025 Jun 23. doi: 10.1038/s41380-025-03079-w. Online ahead of print. Mol Psychiatry. 2025. PMID: 40550863
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

