A multistep in vitro hemocompatibility testing protocol recapitulating the foreign body reaction to nanocarriers
- PMID: 35318565
- PMCID: PMC9360154
- DOI: 10.1007/s13346-022-01141-6
A multistep in vitro hemocompatibility testing protocol recapitulating the foreign body reaction to nanocarriers
Abstract
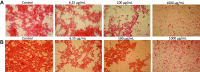
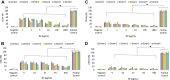
The development of drug nanocarriers based on polymeric, lipid and ceramic biomaterials has been paving the way to precision medicine, where the delivery of poorly soluble active compounds and personalized doses are made possible. However, the nano-size character of these carriers has been demonstrated to have the potential to elicit pathways of the host response different from those of the same biomaterials when engineered as larger size implants and of the drugs when administered without a carrier. Therefore, a specific regulatory framework needs to be made available that can offer robust scientific insights and provide safety data by reliable tests of these novel nano-devices. In this context, the present work presents a multistep protocol for the in vitro assessment of the hemocompatibility of nanocarriers of different physicochemical properties. Poly (ethyl butyl cyanoacrylate) nanoparticles and lipid-based (LipImage™ 815) nanoparticles of comparable hydrodynamic diameter were tested through a battery of assays using human peripheral blood samples and recapitulating the main pathways of the host response upon systemic administration; i.e., protein interactions, fibrinogen-platelet binding, cytotoxicity, and inflammatory response. The data showed the sensitivity and reproducibility of the methods adopted that were also demonstrated to determine individual variability as well as to discriminate between activation of pathways of inflammation and unintended release of inflammatory signaling caused by loss of cell integrity. Therefore, this multistep testing is proposed as a reliable protocol for nanoparticle development and emerging regulatory frameworks.
Keywords: Cytotoxicity; Drug nanocarriers; Hemocompatibility; Host response; In vitro tests; Inflammatory response; Lipid nanoparticles; Nanobiomaterials; Polymeric nanoparticles; Protein corona; Thrombogenicity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Standardization of an in vitro assay matrix to assess cytotoxicity of organic nanocarriers: a pilot interlaboratory comparison.Drug Deliv Transl Res. 2022 Sep;12(9):2187-2206. doi: 10.1007/s13346-022-01203-9. Epub 2022 Jul 6. Drug Deliv Transl Res. 2022. PMID: 35794354 Free PMC article.
-
Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators.Acc Chem Res. 2020 Aug 18;53(8):1545-1556. doi: 10.1021/acs.accounts.0c00280. Epub 2020 Jul 15. Acc Chem Res. 2020. PMID: 32667182
-
Lipid nanocarriers: influence of lipids on product development and pharmacokinetics.Crit Rev Ther Drug Carrier Syst. 2011;28(4):357-93. doi: 10.1615/critrevtherdrugcarriersyst.v28.i4.20. Crit Rev Ther Drug Carrier Syst. 2011. PMID: 21967401 Review.
-
Transdermal Lipid Nanocarriers: A Potential Delivery System for Lornoxicam.Pharm Nanotechnol. 2017;5(1):32-43. doi: 10.2174/2211738505666170105161336. Pharm Nanotechnol. 2017. PMID: 28948909
-
Lipid nanoparticles for dermal drug delivery.Curr Pharm Des. 2015;21(20):2823-9. doi: 10.2174/1381612821666150428143730. Curr Pharm Des. 2015. PMID: 25925115 Review.
Cited by
-
In Vitro Hemocompatibility and Genotoxicity Evaluation of Dual-Labeled [99mTc]Tc-FITC-Silk Fibroin Nanoparticles for Biomedical Applications.Pharmaceuticals (Basel). 2023 Feb 7;16(2):248. doi: 10.3390/ph16020248. Pharmaceuticals (Basel). 2023. PMID: 37259395 Free PMC article.
-
Advances in constructing biocompatible nanocarriers.Drug Deliv Transl Res. 2025 Oct;15(10):3439-3465. doi: 10.1007/s13346-025-01893-x. Epub 2025 Jun 18. Drug Deliv Transl Res. 2025. PMID: 40531369 Review.
-
Standardization of an in vitro assay matrix to assess cytotoxicity of organic nanocarriers: a pilot interlaboratory comparison.Drug Deliv Transl Res. 2022 Sep;12(9):2187-2206. doi: 10.1007/s13346-022-01203-9. Epub 2022 Jul 6. Drug Deliv Transl Res. 2022. PMID: 35794354 Free PMC article.
-
Targeted Drug Administration onto Cancer Cells Using Hyaluronic Acid-Quercetin-Conjugated Silver Nanoparticles.Molecules. 2023 May 17;28(10):4146. doi: 10.3390/molecules28104146. Molecules. 2023. PMID: 37241888 Free PMC article.
-
Synthesis, Characterization and Biocompatibility Evaluation of Novel Chitosan Lipid Micro-Systems for Modified Release of Diclofenac Sodium.Biomedicines. 2023 Feb 4;11(2):453. doi: 10.3390/biomedicines11020453. Biomedicines. 2023. PMID: 36830989 Free PMC article.
References
-
- Mishra RK, Ahmad A , Vyawahare A , j Alam P , Khan TH , Rehan Khan R. Biological effects of formation of protein corona onto nanoparticles. Int J Macromol. 2021;175:1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

