Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician's Review
- PMID: 35318617
- PMCID: PMC8940595
- DOI: 10.1007/s40120-022-00339-7
Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician's Review
Abstract
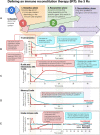
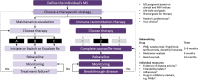
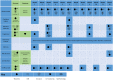
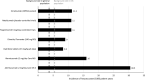
Multiple sclerosis (MS) is a chronic neurodegenerative disease characterized by inflammation and demyelination for which there is currently no cure; therefore, the aim of therapy is to reduce the risk of relapse and disability progression. The treatment options for MS have increased greatly in recent years with the development of several disease-modifying therapies (DMTs) and the advent of immune reconstitution therapy (IRT). IRTs are administered in short-dosing periods to produce long-term effects on the immune system. Treatment with an IRT is based on the 3Rs: reduction, repopulation, and reconstitution of lymphocytes, which leads to restoration of immune effector functions. Cladribine tablets represent a selective, high-efficacy, oral form of IRT for patients with MS that targets lymphocytes and spares innate immune cells. Patients require only two weekly treatment courses, with each course comprising two treatment weeks, in Years 1 and 2; therefore, cladribine tablets are associated with a lower monitoring burden than many other DMTs, while short dosing periods can help to improve adherence. This review provides an overview of IRT and offers the clinician's perspective on the current MS treatment landscape, with a focus on practical advice for the management of patients undergoing treatment with cladribine tablets based on the most recent evidence available, including risks associated with COVID-19 and recommendations for vaccination in patients with MS.
Keywords: Cladribine; Disease-modifying therapy; Immune reconstitution therapy; Multiple sclerosis.
© 2022. The Author(s).
Figures

Similar articles
-
Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis.Mult Scler Relat Disord. 2019 Apr;29:168-174. doi: 10.1016/j.msard.2019.01.038. Epub 2019 Jan 24. Mult Scler Relat Disord. 2019. PMID: 30885375
-
Position of Cladribine Tablets in the Management of Relapsing-Remitting Multiple Sclerosis: An Expert Narrative Review From the United Arab Emirates.Neurol Ther. 2021 Dec;10(2):435-454. doi: 10.1007/s40120-021-00243-6. Epub 2021 Apr 23. Neurol Ther. 2021. PMID: 33891277 Free PMC article. Review.
-
Cladribine Tablets and Relapsing-Remitting Multiple Sclerosis: A Pragmatic, Narrative Review of What Physicians Need to Know.Neurol Ther. 2020 Jun;9(1):11-23. doi: 10.1007/s40120-020-00177-5. Epub 2020 Feb 13. Neurol Ther. 2020. PMID: 32056129 Free PMC article. Review.
-
Real-world experience of cladribine treatment in relapsing-remitting multiple sclerosis: A Danish nationwide study.Mult Scler Relat Disord. 2023 Feb;70:104491. doi: 10.1016/j.msard.2022.104491. Epub 2022 Dec 28. Mult Scler Relat Disord. 2023. PMID: 36623393
-
Immunological consequences of "immune reconstitution therapy" in multiple sclerosis: A systematic review.Autoimmun Rev. 2020 Apr;19(4):102492. doi: 10.1016/j.autrev.2020.102492. Epub 2020 Feb 13. Autoimmun Rev. 2020. PMID: 32062028
Cited by
-
Narrative Review on the Use of Cladribine Tablets as Exit Therapy for Stable Elderly Patients with Multiple Sclerosis.Neurol Ther. 2024 Jun;13(3):519-533. doi: 10.1007/s40120-024-00603-y. Epub 2024 Apr 8. Neurol Ther. 2024. PMID: 38587749 Free PMC article. Review.
-
Update and Application of a Deep Learning Model for the Prediction of Interactions between Drugs Used by Patients with Multiple Sclerosis.Pharmaceutics. 2023 Dec 19;16(1):3. doi: 10.3390/pharmaceutics16010003. Pharmaceutics. 2023. PMID: 38276481 Free PMC article.
-
The Place of Immune Reconstitution Therapy in the Management of Relapsing Multiple Sclerosis in France: An Expert Consensus.Neurol Ther. 2023 Apr;12(2):351-369. doi: 10.1007/s40120-022-00430-z. Epub 2022 Dec 24. Neurol Ther. 2023. PMID: 36564664 Free PMC article. Review.
-
Multicentre Observational Study of Treatment Satisfaction with Cladribine Tablets in the Management of Relapsing Multiple Sclerosis in the Arabian Gulf: The CLUE Study.Neurol Ther. 2023 Aug;12(4):1309-1318. doi: 10.1007/s40120-023-00497-2. Epub 2023 Jun 8. Neurol Ther. 2023. PMID: 37289421 Free PMC article.
-
Administration and Monitoring Burden of High-Efficacy Disease-Modifying Therapies for Multiple Sclerosis: A Delphi Consensus of Clinical Experts from Saudi Arabia.Neurol Ther. 2025 Feb;14(1):413-427. doi: 10.1007/s40120-024-00707-5. Epub 2025 Jan 4. Neurol Ther. 2025. PMID: 39755895 Free PMC article.
References
-
- The Multiple Sclerosis International Federation. Atlas of MS 3rd edn. 2020. https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidem.... Accessed Aug 2021.
Publication types
LinkOut - more resources
Full Text Sources

