Addressing CT metal artifacts using photon-counting detectors and one-step spectral CT image reconstruction
- PMID: 35318699
- PMCID: PMC9353719
- DOI: 10.1002/mp.15621
Addressing CT metal artifacts using photon-counting detectors and one-step spectral CT image reconstruction
Abstract
Purpose: The constrained one-step spectral CT image reconstruction (cOSSCIR) algorithm with a nonconvex alternating direction method of multipliers optimizer is proposed for addressing computed tomography (CT) metal artifacts caused by beam hardening, noise, and photon starvation. The quantitative performance of cOSSCIR is investigated through a series of photon-counting CT simulations.
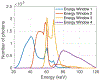
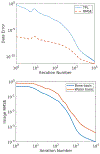
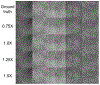
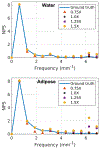
Methods: cOSSCIR directly estimates basis material maps from photon-counting data using a physics-based forward model that accounts for beam hardening. The cOSSCIR optimization framework places constraints on the basis maps, which we hypothesize will stabilize the decomposition and reduce streaks caused by noise and photon starvation. Another advantage of cOSSCIR is that the spectral data need not be registered, so that a ray can be used even if some energy window measurements are unavailable. Photon-counting CT acquisitions of a virtual pelvic phantom with low-contrast soft tissue texture and bilateral hip prostheses were simulated. Bone and water basis maps were estimated using the cOSSCIR algorithm and combined to form a virtual monoenergetic image for the evaluation of metal artifacts. The cOSSCIR images were compared to a "two-step" decomposition approach that first estimated basis sinograms using a maximum likelihood algorithm and then reconstructed basis maps using an iterative total variation constrained least-squares optimization (MLE+TV ). Images were also compared to a nonspectral TV reconstruction of the total number of counts detected for each ray with and without normalized metal artifact reduction (NMAR) applied. The simulated metal density was increased to investigate the effects of increasing photon starvation. The quantitative error and standard deviation in regions of the phantom were compared across the investigated algorithms. The ability of cOSSCIR to reproduce the soft-tissue texture, while reducing metal artifacts, was quantitatively evaluated.
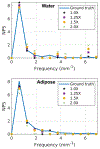
Results: Noiseless simulations demonstrated the convergence of the cOSSCIR and MLE+TV algorithms to the correct basis maps in the presence of beam-hardening effects. When noise was simulated, cOSSCIR demonstrated a quantitative error of -1 HU, compared to 2 HU error for the MLE+TV algorithm and -154 HU error for the nonspectral TV +NMAR algorithm. For the cOSSCIR algorithm, the standard deviation in the central iodine region of interest was 20 HU, compared to 299 HU for the MLE+TV algorithm, 41 HU for the MLE+TV +Mask algorithm that excluded rays through metal, and 55 HU for the nonspectral TV +NMAR algorithm. Increasing levels of photon starvation did not impact the bias or standard deviation of the cOSSCIR images. cOSSCIR was able to reproduce the soft-tissue texture when an appropriate regularization constraint value was selected.
Conclusions: By directly inverting photon-counting CT data into basis maps using an accurate physics-based forward model and a constrained optimization algorithm, cOSSCIR avoids metal artifacts due to beam hardening, noise, and photon starvation. The cOSSCIR algorithm demonstrated improved stability and accuracy compared to a two-step method of decomposition followed by reconstruction.
Keywords: metal artifacts; photon-counting CT; reconstruction.
© 2022 American Association of Physicists in Medicine.
Figures










Similar articles
-
Constrained one-step material decomposition reconstruction of head CT data from a silicon photon-counting prototype.Med Phys. 2023 Oct;50(10):6008-6021. doi: 10.1002/mp.16649. Epub 2023 Jul 31. Med Phys. 2023. PMID: 37523258 Free PMC article.
-
Dual energy CT reconstruction using the constrained one step spectral image reconstruction algorithm.Med Phys. 2024 Apr;51(4):2648-2664. doi: 10.1002/mp.16788. Epub 2023 Oct 14. Med Phys. 2024. PMID: 37837648 Free PMC article.
-
Photon-counting normalized metal artifact reduction (NMAR) in diagnostic CT.Med Phys. 2021 Jul;48(7):3572-3582. doi: 10.1002/mp.14931. Epub 2021 May 28. Med Phys. 2021. PMID: 33973237
-
[Incident Photon Number and Reconstructed Linear Attenuation Coefficients in Iterative CT Image Reconstruction].Igaku Butsuri. 2019;38(4):143-158. doi: 10.11323/jjmp.38.4_143. Igaku Butsuri. 2019. PMID: 30828046 Review. Japanese.
-
Dual-Energy CT for Pediatric Thoracic Imaging: A Review.AJR Am J Roentgenol. 2023 Oct;221(4):526-538. doi: 10.2214/AJR.23.29244. Epub 2023 May 17. AJR Am J Roentgenol. 2023. PMID: 37195790 Review.
Cited by
-
Simultaneous activity and attenuation estimation in TOF-PET with TV-constrained nonconvex optimization.ArXiv [Preprint]. 2024 Feb 9:arXiv:2303.17042v2. ArXiv. 2024. Update in: IEEE Trans Med Imaging. 2024 Jun;43(6):2347-2357. doi: 10.1109/TMI.2024.3365302. PMID: 37033460 Free PMC article. Updated. Preprint.
-
Assessment of material identification and quantification in the presence of metals using spectral photon counting CT.PLoS One. 2024 Sep 13;19(9):e0308658. doi: 10.1371/journal.pone.0308658. eCollection 2024. PLoS One. 2024. PMID: 39269959 Free PMC article.
-
Spectral calibration of photon-counting detectors at high photon flux.Med Phys. 2022 Oct;49(10):6368-6383. doi: 10.1002/mp.15942. Epub 2022 Aug 31. Med Phys. 2022. PMID: 35975670 Free PMC article.
-
Direct Multi-Material Reconstruction via Iterative Proximal Adaptive Descent for Spectral CT Imaging.Bioengineering (Basel). 2023 Apr 12;10(4):470. doi: 10.3390/bioengineering10040470. Bioengineering (Basel). 2023. PMID: 37106656 Free PMC article.
-
Approaches, advantages, and challenges to photon counting detector and multi-energy CT.Abdom Radiol (NY). 2024 Sep;49(9):3251-3260. doi: 10.1007/s00261-024-04357-x. Epub 2024 May 15. Abdom Radiol (NY). 2024. PMID: 38744702 Review.
References
-
- Gjesteby L, De Man B, Jin Y, Paganetti H, Verburg J, Giantsoudi D, and Wang G, Metal Artifact Reduction in CT: Where Are We After Four Decades?, IEEE Access 4, 5826–5849 (2016).
-
- Wellenberg R, Hakvoort E, Slump C, Boomsma M, Maas M, and Streekstra G, Metal artifact reduction techniques in musculoskeletal CT-imaging, European Journal of Radiology 107, 60–69 (2018). - PubMed
-
- Han SC, Chung YE, Lee YH, Park KK, Kim MJ, and Kim KW, Metal artifact reduction software used with abdominopelvic dual-energy CT of patients with metal hip prostheses: assessment of image quality and clinical feasibility, American Journal of Roentgenology 203, 788–795 (2014). - PubMed
-
- Axente M, Paidi A, Von Eyben R, Zeng C, Bani-Hashemi A, Krauss A, and Hristov D, Clinical evaluation of the iterative metal artifact reduction algorithm for CT simulation in radiotherapy, Medical physics 42, 1170–1183 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

