Aerosolized vitamin E acetate causes oxidative injury in mice and in alveolar macrophages
- PMID: 35318859
- PMCID: PMC9109788
- DOI: 10.1152/ajplung.00482.2021
Aerosolized vitamin E acetate causes oxidative injury in mice and in alveolar macrophages
Abstract
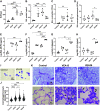
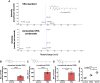
Although vitamin E acetate (VEA) is suspected to play a causal role in the development of electronic-cigarette, or vaping, product use-associated lung injury (EVALI), the underlying biological mechanisms of pulmonary injury are yet to be determined. In addition, no study has replicated the systemic inflammation observed in humans in a murine EVALI model, nor investigated potential additive toxicity of viral infection in the setting of exposure to vaping products. To identify the mechanisms driving VEA-related lung injury and test the hypothesis that viral infection causes additive lung injury in the presence of aerosolized VEA, we exposed mice to aerosolized VEA for extended times, followed by influenza infection in some experiments. We used mass spectrometry to evaluate the composition of aerosolized VEA condensate and the VEA deposition in murine or human alveolar macrophages. Extended vaping for 28 days versus 15 days did not worsen lung injury but caused systemic inflammation in the murine EVALI model. Vaping plus influenza increased lung water compared with virus alone. Murine alveolar macrophages exposed to vaped VEA hydrolyzed the VEA to vitamin E with evidence of oxidative stress in the alveolar space and systemic circulation. Aerosolized VEA also induced cell death and chemokine release and reduced efferocytotic function in human alveolar macrophages in vitro. These findings provide new insights into the biological mechanisms of VEA toxicity.
Keywords: E-cigarette or vaping product use-associated lung injury; acute respiratory distress syndrome; alveolar macrophage; oxidative stress; vitamin E acetate.
Conflict of interest statement
C. S. Calfee has received grants and personal fees from Bayer and Roche-Genentech, and personal fees from Quark Pharmaceuticals, Vasomune, Gen1e Life Sciences; and Janssen. M. A. Matthay has received grants from Roche-Genentech and Bayer Pharmaceuticals, personal fees from Boehringer Ingelheim, Novartis Pharmaceuticals, Citius Pharmaceuticals, Pliant Therapeutics, Johnson & Johnson Pharmaceuticals, and Gilead Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




Similar articles
-
A mouse model of E-cigarette or vaping product use-associated lung injury (EVALI) induced by nose-only exposure to aerosolized vitamin E acetate and associated macrophage dysfunction.Respir Res. 2025 Aug 31;26(1):263. doi: 10.1186/s12931-025-03343-1. Respir Res. 2025. PMID: 40887642 Free PMC article.
-
Dose-Dependent Pulmonary Toxicity of Aerosolized Vitamin E Acetate.Am J Respir Cell Mol Biol. 2020 Dec;63(6):748-757. doi: 10.1165/rcmb.2020-0209OC. Am J Respir Cell Mol Biol. 2020. PMID: 32822237 Free PMC article.
-
Compiling Evidence for EVALI: A Scoping Review of In Vivo Pulmonary Effects After Inhaling Vitamin E or Vitamin E Acetate.J Med Toxicol. 2021 Jul;17(3):278-288. doi: 10.1007/s13181-021-00823-w. Epub 2021 Feb 2. J Med Toxicol. 2021. PMID: 33528766 Free PMC article.
-
E-cigarette vaping associated acute lung injury (EVALI): state of science and future research needs.Crit Rev Toxicol. 2022 Mar;52(3):188-220. doi: 10.1080/10408444.2022.2082918. Epub 2022 Jul 13. Crit Rev Toxicol. 2022. PMID: 35822508 Free PMC article. Review.
-
Comparative study of lung toxicity of E-cigarette ingredients to investigate E-cigarette or vaping product associated lung injury.J Hazard Mater. 2023 Mar 5;445:130454. doi: 10.1016/j.jhazmat.2022.130454. Epub 2022 Dec 5. J Hazard Mater. 2023. PMID: 37055947
Cited by
-
Multi-omic assessment shows dysregulation of pulmonary and systemic immunity to e-cigarette exposure.Respir Res. 2023 May 25;24(1):138. doi: 10.1186/s12931-023-02441-2. Respir Res. 2023. PMID: 37231407 Free PMC article.
-
Comparison of emissions across tobacco products: A slippery slope in tobacco control.Tob Induc Dis. 2024 Mar 30;22. doi: 10.18332/tid/183797. eCollection 2024. Tob Induc Dis. 2024. PMID: 38560551 Free PMC article. Review.
-
Chronic airway inflammatory diseases and e-cigarette use: a review of health risks and mechanisms.Eur J Med Res. 2025 Apr 1;30(1):223. doi: 10.1186/s40001-025-02492-9. Eur J Med Res. 2025. PMID: 40170170 Free PMC article. Review.
-
E-cigarette or vaping product use-associated lung injury (EVALI). A case report of a 19-year-old male in Denmark.Eur Clin Respir J. 2025 Jan 3;12(1):2445868. doi: 10.1080/20018525.2024.2445868. eCollection 2025. Eur Clin Respir J. 2025. PMID: 39764358 Free PMC article.
-
The implications of Vitamin E acetate in E-cigarette, or vaping, product use-associated lung injury.Ann Thorac Med. 2023 Jan-Mar;18(1):1-9. doi: 10.4103/atm.atm_144_22. Epub 2023 Jan 25. Ann Thorac Med. 2023. PMID: 36968330 Free PMC article. Review.
References
-
- Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—preliminary report. New Engl J Med 382: 903–916, 2020. doi:10.1056/NEJMoa1911614. - DOI - PubMed
-
- Schier JG, Meiman JG, Layden J, Mikosz CA, VanFrank B, King BA, Salvatore PP, Weissman DN, Thomas J, Melstrom PC, Baldwin GT, Parker EM, Courtney-Long EA, Krishnasamy VP, Pickens CM, Evans ME, Tsay SV, Powell KM, Kiernan EA, Marynak KL, Adjemian J, Holton K, Armour BS, England LJ, Briss PA, Houry D, Hacker KA, Reagan-Steiner S, Zaki S, Meaney-Delman D; CDC 2019 Lung Injury Response Group. Severe pulmonary disease associated with electronic-cigarette-product use - interim guidance. MMWR Morb Mortal Wkly Rep 68: 787–790, 2019. [Erratum in MMWR Morb Mortal Wkly Rep 68: 830, 2019]. doi:10.15585/mmwr.mm6836e2. - DOI - PMC - PubMed
-
- Matthay MA, Arabi YM, Siegel ER, Ware LB, Bos LDJ, Sinha P, Beitler JR, Wick KD, Curley MAQ, Constantin JM, Levitt JE, Calfee CS. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med 46: 2136–2152, 2020. doi:10.1007/s00134-020-06296-9. - DOI - PMC - PubMed
-
- Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion. Outbreak of Lung Injury Associated With the Use Of E-Cigarette, or Vaping, Products (Online). Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-d... [2022 Feb 14].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

