Decrease in Tuberculosis Cases during COVID-19 Pandemic as Reflected by Outpatient Pharmacy Data, United States, 2020
- PMID: 35318920
- PMCID: PMC8962886
- DOI: 10.3201/eid2804.212014
Decrease in Tuberculosis Cases during COVID-19 Pandemic as Reflected by Outpatient Pharmacy Data, United States, 2020
Abstract
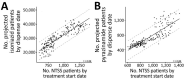
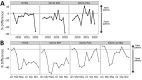
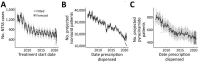
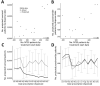
We analyzed a pharmacy dataset to assess the 20% decline in tuberculosis (TB) cases reported to the US National Tuberculosis Surveillance System (NTSS) during the coronavirus disease pandemic in 2020 compared with the 2016-2019 average. We examined the correlation between TB medication dispensing data to TB case counts in NTSS and used a seasonal autoregressive integrated moving average model to predict expected 2020 counts. Trends in the TB medication data were correlated with trends in NTSS data during 2006-2019. There were fewer prescriptions and cases in 2020 than would be expected on the basis of previous trends. This decrease was particularly large during April-May 2020. These data are consistent with NTSS data, suggesting that underreporting is not occurring but not ruling out underdiagnosis or actual decline. Understanding the mechanisms behind the 2020 decline in reported TB cases will help TB programs better prepare for postpandemic cases.
Keywords: COVID-19; SARIMA; SARS-CoV-2; United States; bacteria; coronavirus disease; prescriptions; respiratory infections; severe acute respiratory syndrome coronavirus 2; surveillance; tuberculosis and other mycobacteria; viruses; zoonoses.
Figures
References
-
- Centers for Disease Control and Prevention (CDC). Reported tuberculosis in the United States, 2019. [cited 2021 Jul 27]. https://www.cdc.gov/tb/statistics/reports/2019/default.htm
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

