Unique Clinical, Immune, and Genetic Signature in Patients with Borrelial Meningoradiculoneuritis1
- PMID: 35318928
- PMCID: PMC8962912
- DOI: 10.3201/eid2804.211831
Unique Clinical, Immune, and Genetic Signature in Patients with Borrelial Meningoradiculoneuritis1
Abstract
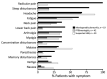
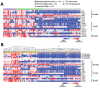
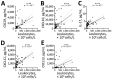
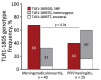
Lyme neuroborreliosis (LNB) in Europe may manifest with painful meningoradiculoneuritis (also known as Bannwarth syndrome) or lymphocytic meningitis with or without cranial neuritis (peripheral facial palsy). We assessed host immune responses and the prevalence of TLR1 (toll-like receptor 1)-1805GG polymorphism to gain insights into the pathophysiology of these conditions. Regardless of LNB manifestation, most mediators associated with innate and adaptive immune responses were concentrated in cerebrospinal fluid; serum levels were unremarkable. When stratified by specific clinical manifestation, patients with meningoradiculoneuritis had higher levels of B-cell chemoattractants CXC motif chemokine ligand (CXCL) 12 and CXCL13 and T-cell-associated mediators CXCL9, CXCL10, and interleukin 17, compared with those without radicular pain. Moreover, these patients had a higher frequency of TLR1-1805GG polymorphism and more constitutional symptoms. These findings demonstrate that meningoradiculoneuritis is a distinct clinical entity with unique immune and genetic pathophysiology, providing new considerations for the study of LNB and borrelial meningoradiculitis.
Keywords: Lyme disease; Slovenia; United States; bacteria; cytokines; genetics; immune response; inflammation; meningitis/encephalitis; meningoradiculoneuritis; neuroborreliosis; peripheral facial palsy; polymorphism; vector-borne infections.
Figures


Similar articles
-
Proportion of confirmed Lyme neuroborreliosis cases among adult patients with suspected early European Lyme neuroborreliosis.Infection. 2025 Aug;53(4):1403-1412. doi: 10.1007/s15010-024-02461-0. Epub 2025 Jan 2. Infection. 2025. PMID: 39747734 Free PMC article.
-
Why Is the Duration of Erythema Migrans at Diagnosis Longer in Patients with Lyme Neuroborreliosis Than in Those without Neurologic Involvement?Pathogens. 2024 Feb 1;13(2):137. doi: 10.3390/pathogens13020137. Pathogens. 2024. PMID: 38392875 Free PMC article.
-
Lyme neuroborreliosis.Handb Clin Neurol. 2013;115:559-75. doi: 10.1016/B978-0-444-52902-2.00032-1. Handb Clin Neurol. 2013. PMID: 23931802 Review.
-
Clinical manifestations and long-term outcome of early Lyme neuroborreliosis according to the European Federation of Neurological Societies diagnostic criteria (definite versus possible) in central Europe. A retrospective cohort study.Eur J Neurol. 2021 Sep;28(9):3155-3166. doi: 10.1111/ene.14962. Epub 2021 Jul 1. Eur J Neurol. 2021. PMID: 34114701
-
Clinical manifestations of Lyme neuroborreliosis in children: a review.Eur J Pediatr. 2023 May;182(5):1965-1976. doi: 10.1007/s00431-023-04811-w. Epub 2023 Mar 1. Eur J Pediatr. 2023. PMID: 36856886 Review.
Cited by
-
Lower Frequency of Multiple Erythema Migrans Skin Lesions in Lyme Reinfections, Europe.Emerg Infect Dis. 2025 Apr;31(4):662-668. doi: 10.3201/eid3104.241329. Emerg Infect Dis. 2025. PMID: 40133040 Free PMC article. Review.
-
Intrathecal Th17-driven inflammation is associated with prolonged post-treatment convalescence for patients with Lyme neuroborreliosis.Sci Rep. 2023 Jun 15;13(1):9722. doi: 10.1038/s41598-023-36709-w. Sci Rep. 2023. PMID: 37322136 Free PMC article.
-
Central nervous system manifestations in rheumatic diseases.Rheumatol Int. 2024 Oct;44(10):1803-1812. doi: 10.1007/s00296-024-05679-1. Epub 2024 Aug 13. Rheumatol Int. 2024. PMID: 39136787 Review.
-
Cerebrospinal fluid protein profiling of inflammatory and neurobiological markers in Lyme neuroborreliosis.Sci Rep. 2025 Jun 20;15(1):20190. doi: 10.1038/s41598-025-06146-y. Sci Rep. 2025. PMID: 40542080 Free PMC article.
References
-
- Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010;17:8–16, e1–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

