Heparanase Blockade as a Novel Dual-Targeting Therapy for COVID-19
- PMID: 35319225
- PMCID: PMC9006938
- DOI: 10.1128/jvi.00057-22
Heparanase Blockade as a Novel Dual-Targeting Therapy for COVID-19
Abstract
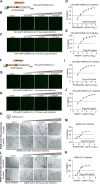
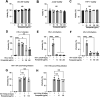
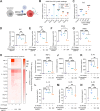
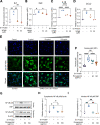
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused over 5 million deaths worldwide. Pneumonia and systemic inflammation contribute to its high mortality. Many viruses use heparan sulfate proteoglycans as coreceptors for viral entry, and heparanase (HPSE) is a known regulator of both viral entry and inflammatory cytokines. We evaluated the heparanase inhibitor Roneparstat, a modified heparin with minimum anticoagulant activity, in pathophysiology and therapy for COVID-19. We found that Roneparstat significantly decreased the infectivity of SARS-CoV-2, SARS-CoV-1, and retroviruses (human T-lymphotropic virus 1 [HTLV-1] and HIV-1) in vitro. Single-cell RNA sequencing (scRNA-seq) analysis of cells from the bronchoalveolar lavage fluid of COVID-19 patients revealed a marked increase in HPSE gene expression in CD68+ macrophages compared to healthy controls. Elevated levels of HPSE expression in macrophages correlated with the severity of COVID-19 and the expression of inflammatory cytokine genes, including IL6, TNF, IL1B, and CCL2. In line with this finding, we found a marked induction of HPSE and numerous inflammatory cytokines in human macrophages challenged with SARS-CoV-2 S1 protein. Treatment with Roneparstat significantly attenuated SARS-CoV-2 S1 protein-mediated inflammatory cytokine release from human macrophages, through disruption of NF-κB signaling. HPSE knockdown in a macrophage cell line also showed diminished inflammatory cytokine production during S1 protein challenge. Taken together, this study provides a proof of concept that heparanase is a target for SARS-CoV-2-mediated pathogenesis and that Roneparstat may serve as a dual-targeted therapy to reduce viral infection and inflammation in COVID-19. IMPORTANCE The complex pathogenesis of COVID-19 consists of two major pathological phases: an initial infection phase elicited by SARS-CoV-2 entry and replication and an inflammation phase that could lead to tissue damage, which can evolve into acute respiratory failure or even death. While the development and deployment of vaccines are ongoing, effective therapy for COVID-19 is still urgently needed. In this study, we explored HPSE blockade with Roneparstat, a phase I clinically tested HPSE inhibitor, in the context of COVID-19 pathogenesis. Treatment with Roneparstat showed wide-spectrum anti-infection activities against SARS-CoV-2, HTLV-1, and HIV-1 in vitro. In addition, HPSE blockade with Roneparstat significantly attenuated SARS-CoV-2 S1 protein-induced inflammatory cytokine release from human macrophages through disruption of NF-κB signaling. Together, this study provides a proof of principle for the use of Roneparstat as a dual-targeting therapy for COVID-19 to decrease viral infection and dampen the proinflammatory immune response mediated by macrophages.
Keywords: COVID-19; SARS-CoV-2; heparanase; inflammatory cytokine release; macrophage.
Conflict of interest statement
The authors declare a conflict of interest. Roneparstat (SST0001) is a proprietary drug of Leadiant Biosciences S.p.A. Alessandro Noseda is the employee of Leadiant Biosciences S.p.A. No potential conflicts of interest were disclosed by the other authors.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

