Deep-Time Structural Evolution of Retroviral and Filoviral Surface Envelope Proteins
- PMID: 35319227
- PMCID: PMC9006886
- DOI: 10.1128/jvi.00063-22
Deep-Time Structural Evolution of Retroviral and Filoviral Surface Envelope Proteins
Abstract
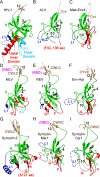
The retroviral surface envelope protein subunit (SU) mediates receptor binding and triggers membrane fusion by the transmembrane (TM) subunit. SU evolves rapidly under strong selective conditions, resulting in seemingly unrelated SU structures in highly divergent retroviruses. Structural modeling of the SUs of several retroviruses and related endogenous retroviral elements with AlphaFold 2 identifies a TM-proximal SU β-sandwich structure that has been conserved in the orthoretroviruses for at least 110 million years. The SU of orthoretroviruses diversified by the differential expansion of the β-sandwich core to form domains involved in virus-host interactions. The β-sandwich domain is also conserved in the SU equivalent GP1 of Ebola virus although with a significantly different orientation in the trimeric envelope protein structure relative to the β-sandwich of human immunodeficiency virus type 1 gp120, with significant evidence for divergent rather than convergent evolution. The unified structural view of orthoretroviral SU and filoviral GP1 identifies an ancient, structurally conserved, and evolvable domain underlying the structural diversity of orthoretroviral SU and filoviral GP1. IMPORTANCE The structural relationships of SUs of retroviral groups are obscured by the high rate of sequence change of SU and the deep-time divergence of retroviral lineages. Previous data showed no structural or functional relationships between the SUs of type C gammaretroviruses and lentiviruses. A deeper understanding of structural relationships between the SUs of different retroviral lineages would allow the generalization of critical processes mediated by these proteins in host cell infection. Modeling of SUs with AlphaFold 2 reveals a conserved core domain underlying the structural diversity of orthoretroviral SUs. Definition of the conserved SU structural core allowed the identification of a homologue structure in the SU equivalent GP1 of filoviruses that most likely shares an origin, unifying the SU of orthoretroviruses and GP1 of filoviruses into a single protein family. These findings will allow an understanding of the structural basis for receptor-mediated membrane fusion mechanisms in a broad range of biomedically important retroviruses.
Keywords: ALV; EBOV; FELV; GP1; HIV-1; MLV; RBD; alpharetrovirus; betaretrovirus; foamy virus; gammaretrovirus; gp120; lentivirus; spumaretrovirus; syncytin.
Conflict of interest statement
The authors declare a conflict of interest. The author is an Employee of Genentech and holds shares in Roche.
Figures






Similar articles
-
Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review.
-
Domain Organization of Lentiviral and Betaretroviral Surface Envelope Glycoproteins Modeled with AlphaFold.J Virol. 2022 Jan 26;96(2):e0134821. doi: 10.1128/JVI.01348-21. Epub 2021 Oct 27. J Virol. 2022. PMID: 34705555 Free PMC article.
-
Caprine arthritis-encephalitis virus envelope surface glycoprotein regions interacting with the transmembrane glycoprotein: structural and functional parallels with human immunodeficiency virus type 1 gp120.J Virol. 2003 Nov;77(21):11578-87. doi: 10.1128/jvi.77.21.11578-11587.2003. J Virol. 2003. PMID: 14557643 Free PMC article.
-
Conservation of human immunodeficiency virus type 1 gp120 inner-domain sequences in lentivirus and type A and B retrovirus envelope surface glycoproteins.J Virol. 2001 Feb;75(4):2014-8. doi: 10.1128/JVI.75.4.2014-2018.2001. J Virol. 2001. PMID: 11160703 Free PMC article.
-
Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies.Prog Mol Biol Transl Sci. 2017;145:111-162. doi: 10.1016/bs.pmbts.2016.12.005. Epub 2017 Jan 16. Prog Mol Biol Transl Sci. 2017. PMID: 28110749 Review.
Cited by
-
Using AlphaFold Predictions in Viral Research.Curr Issues Mol Biol. 2023 Apr 21;45(4):3705-3732. doi: 10.3390/cimb45040240. Curr Issues Mol Biol. 2023. PMID: 37185764 Free PMC article. Review.
-
Neutralization of zoonotic retroviruses by human antibodies: Genotype-specific epitopes within the receptor-binding domain from simian foamy virus.PLoS Pathog. 2023 Apr 24;19(4):e1011339. doi: 10.1371/journal.ppat.1011339. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37093892 Free PMC article.
-
Unique Structure and Distinctive Properties of the Ancient and Ubiquitous Gamma-Type Envelope Glycoprotein.Viruses. 2023 Jan 18;15(2):274. doi: 10.3390/v15020274. Viruses. 2023. PMID: 36851488 Free PMC article. Review.
-
Receptor-recognition and antiviral mechanisms of retrovirus-derived human proteins.Nat Struct Mol Biol. 2024 Sep;31(9):1368-1376. doi: 10.1038/s41594-024-01295-6. Epub 2024 Apr 26. Nat Struct Mol Biol. 2024. PMID: 38671230
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

