GATA3 Inhibits Viral Infection by Promoting MicroRNA-155 Expression
- PMID: 35319228
- PMCID: PMC9006930
- DOI: 10.1128/jvi.01888-21
GATA3 Inhibits Viral Infection by Promoting MicroRNA-155 Expression
Abstract
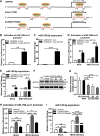
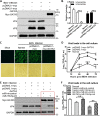
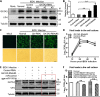
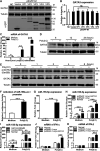
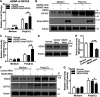
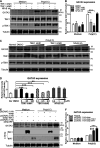
Recognition of viral RNAs by melanoma differentiation associated gene-5 (MDA5) initiates chicken antiviral response by producing type I interferons. Our previous studies showed that chicken microRNA-155-5p (gga-miR-155-5p) enhanced IFN-β expression and suppressed the replication of infectious burse disease virus (IBDV), a double-stranded RNA (dsRNA) virus causing infectious burse disease in chickens. However, the mechanism underlying IBDV-induced gga-miR-155-5p expression in host cells remains elusive. Here, we show that IBDV infection or poly(I:C) treatment of DF-1 cells markedly increased the expression of GATA-binding protein 3 (GATA3), a master regulator for TH2 cell differentiation, and that GATA3 promoted gga-miR-155-5p expression in IBDV-infected or poly(I:C)-treated cells by directly binding to its promoter. Surprisingly, ectopic expression of GATA3 significantly reduced IBDV replication in DF-1 cells, and this reduction could be completely abolished by treatment with gga-miR-155-5p inhibitors, whereas knockdown of GATA3 by RNA interference enhanced IBDV growth, and this enhancement could be blocked with gga-miR-155-5p mimics, indicating that GATA3 suppressed IBDV replication by gga-miR-155-5p. Furthermore, our data show that MDA5 is required for GATA3 expression in host cells with poly(I:C) treatment, so are the adaptor protein TBK1 and transcription factor IRF7, suggesting that induction of GATA3 expression in IBDV-infected cells relies on MDA5-TBK1-IRF7 signaling pathway. These results uncover a novel role for GATA3 as an antivirus transcription factor in innate immune response by promoting miR-155 expression, further our understandings of host response against pathogenic infection, and provide valuable clues to the development of antiviral reagents for public health. IMPORTANCE Gga-miR-155-5p acts as an important antivirus factor against IBDV infection, which causes a severe immunosuppressive disease in chicken. Elucidation of the mechanism regulating gga-miR-155-5p expression in IBDV-infected cells is essential to our understandings of the host response against pathogenic infection. This study shows that transcription factor GATA3 initiated gga-miR-155-5p expression in IBDV-infected cells by directly binding to its promoter, suppressing viral replication. Furthermore, induction of GATA3 expression was attributable to the recognition of dsRNA by MDA5, which initiates signal transduction via TBK1 and IRF7. Thus, it is clear that IBDV induces GATA3 expression via MDA5-TBK1-IRF7 signaling pathway, thereby suppressing IBDV replication by GATA3-mediated gga-miR-155-5p expression. This information remarkably expands our knowledge of the roles for GATA3 as an antivirus transcription factor in host innate immune response particularly at an RNA level and may prove valuable in the development of antiviral drugs for public health.
Keywords: GATA3; IBDV infection; miR-155.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Epigenetic Upregulation of Chicken MicroRNA-16-5p Expression in DF-1 Cells following Infection with Infectious Bursal Disease Virus (IBDV) Enhances IBDV-Induced Apoptosis and Viral Replication.J Virol. 2020 Jan 6;94(2):e01724-19. doi: 10.1128/JVI.01724-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694944 Free PMC article.
-
gga-miR-142-5p attenuates IRF7 signaling and promotes replication of IBDV by directly targeting the chMDA5's 3' untranslated region.Vet Microbiol. 2018 Jul;221:74-80. doi: 10.1016/j.vetmic.2018.05.018. Epub 2018 May 30. Vet Microbiol. 2018. PMID: 29981711
-
gga-miR-155 Enhances Type I Interferon Expression and Suppresses Infectious Burse Disease Virus Replication via Targeting SOCS1 and TANK.Front Cell Infect Microbiol. 2018 Mar 7;8:55. doi: 10.3389/fcimb.2018.00055. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29564226 Free PMC article.
-
Role of MicroRNAs in Host Defense against Infectious Bursal Disease Virus (IBDV) Infection: A Hidden Front Line.Viruses. 2020 May 14;12(5):543. doi: 10.3390/v12050543. Viruses. 2020. PMID: 32423052 Free PMC article. Review.
-
Infectious bursal disease virus of chickens: pathogenesis and immunosuppression.Dev Comp Immunol. 2000 Mar-Apr;24(2-3):223-35. doi: 10.1016/s0145-305x(99)00074-9. Dev Comp Immunol. 2000. PMID: 10717289 Review.
Cited by
-
Idiopathic inflammatory myopathy and non-coding RNA.Front Immunol. 2023 Sep 6;14:1227945. doi: 10.3389/fimmu.2023.1227945. eCollection 2023. Front Immunol. 2023. PMID: 37744337 Free PMC article. Review.
-
Identification of the T-box transcription factor t-bet in chickens and its role in immune response.Poult Sci. 2025 Jul 19;104(10):105589. doi: 10.1016/j.psj.2025.105589. Online ahead of print. Poult Sci. 2025. PMID: 40737904 Free PMC article.
-
Reduced NR2F2 Expression in the Host Response to Infectious Bursal Disease Virus Infection Suppressed Viral Replication by Enhancing Type I Interferon Expression by Targeting SOCS5.J Virol. 2023 Jul 27;97(7):e0066423. doi: 10.1128/jvi.00664-23. Epub 2023 Jun 26. J Virol. 2023. PMID: 37358466 Free PMC article.
-
Immune mechanisms affected by cyclooxygenase inhibition combined with antiviral treatment in calves infected with bovine respiratory syncytial virus.PLoS One. 2025 Apr 22;20(4):e0321642. doi: 10.1371/journal.pone.0321642. eCollection 2025. PLoS One. 2025. PMID: 40261882 Free PMC article.
-
Exosome derived from tumor-associated macrophages: biogenesis, functions, and therapeutic implications in human cancers.Biomark Res. 2023 Nov 19;11(1):100. doi: 10.1186/s40364-023-00538-w. Biomark Res. 2023. PMID: 37981718 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

