Thyroid dermopathy responds to teprotumumab therapy
- PMID: 35319490
- PMCID: PMC9002184
- DOI: 10.1530/EDM-21-0201
Thyroid dermopathy responds to teprotumumab therapy
Abstract
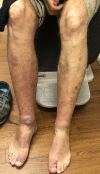
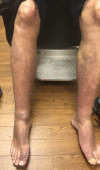
Summary: Thyroid dermopathy is an uncommon manifestation of thyroid disease that impairs the quality of life in certain cases. Currently, the available treatments offer limited results and a chance of recurrence. Teprotumumab, a novel medication that results in the regression of thyroid ophthalmopathy, may have similar effects on dermopathy. We describe four patients treated with teprotumumab for their thyroid ophthalmopathy who concomitantly had dermatopathy upon initiation of their infusions. Patients improved after two to three infusions and three out of the four patients have not suffered a recurrence.Teprotumumab is a monoclonal antibody (MAB) that attenuates an inflammatory response, resulting in decreased edema and tissue expansion. Given the similarities of their pathophysiology, we believe that the resolution of thyroid dermatopathy and regression of thyroid eye disease occurs via the same mechanism. We encourage further investigation utilizing teprotumumab for patients whose dermopathy is associated with impaired quality of life.
Learning points: Thyroid dermopathy (TD), an uncommon manifestation of thyroid disease, may occasionally impair function and quality of life. There are only a few treatments for TD, with limited results and high rates of recurrence. Teprotumumab is a Food and Drug Administration-approved medication used for thyroid eye disease (TED). Our patients treated with teprotumumab for TED showed improvement of TD, which demonstrates its potential use for this condition.
Figures
References
-
- Krieger CC, Neumann S, Place RF, Marcus-Samuels B, Gershengorn MC. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. Journal of Clinical Endocrinology and Metabolism 20151001071–1077. (10.1210/jc.2014-3566) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

