Improving Enrollment of Underrepresented Racial and Ethnic Populations in Heart Failure Trials: A Call to Action From the Heart Failure Collaboratory
- PMID: 35319725
- PMCID: PMC9098689
- DOI: 10.1001/jamacardio.2022.0161
Improving Enrollment of Underrepresented Racial and Ethnic Populations in Heart Failure Trials: A Call to Action From the Heart Failure Collaboratory
Abstract
Importance: Despite bearing a disproportionate burden of heart failure (HF), Black and Hispanic individuals have been poorly represented in HF clinical trials. Underrepresentation in clinical trials limits the generalizability of the findings to these populations and may even introduce uncertainties and hesitancy when translating trial data to the care of people from underrepresented groups. The Heart Failure Collaboratory, a consortium of stakeholders convened to enhance HF therapeutic development, has been dedicated to improving recruitment strategies for patients from diverse and historically underrepresented groups.
Observations: Despite federal policies from the US Food and Drug Administration and National Institutes of Health aimed at improving trial representation, gaps in trial enrollment proportionate to the racial and ethnic composition of the HF population have persisted. Increasing trial globalization with limited US enrollment is a major driver of these patterns. Additional barriers to representative enrollment include inequities in care access, logistical issues in participation, restrictive enrollment criteria, and English language requirements.
Conclusions and relevance: Strategies for improving diverse trial enrollment include methodical study design and site selection, diversification of research leadership and staff, broadening of eligibility criteria, community and patient engagement, and broad stakeholder commitment. In contemporary HF trials, diverse trial enrollment is not only feasible but can be efficiently achieved to improve the generalizability and translation of trial knowledge to clinical practice.
Figures
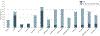
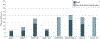

Similar articles
-
Enrollment of Older Patients, Women, and Racial and Ethnic Minorities in Contemporary Heart Failure Clinical Trials: A Systematic Review.JAMA Cardiol. 2018 Oct 1;3(10):1011-1019. doi: 10.1001/jamacardio.2018.2559. JAMA Cardiol. 2018. PMID: 30140928
-
Eligibility criteria and enrollment of a diverse racial and ethnic population in multiple myeloma clinical trials.Blood. 2023 Jul 20;142(3):235-243. doi: 10.1182/blood.2022018657. Blood. 2023. PMID: 37140031
-
Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 May 3;4(5):e218348. doi: 10.1001/jamanetworkopen.2021.8348. JAMA Netw Open. 2021. PMID: 34003274 Free PMC article.
-
Enrollment of Older Patients, Women, and Racial/Ethnic Minority Groups in Contemporary Acute Coronary Syndrome Clinical Trials: A Systematic Review.JAMA Cardiol. 2020 Jun 1;5(6):714-722. doi: 10.1001/jamacardio.2020.0359. JAMA Cardiol. 2020. PMID: 32211813
-
Racial/Ethnic Disparities in Ophthalmology Clinical Trials Resulting in US Food and Drug Administration Drug Approvals From 2000 to 2020.JAMA Ophthalmol. 2021 Jun 1;139(6):629-637. doi: 10.1001/jamaophthalmol.2021.0857. JAMA Ophthalmol. 2021. PMID: 33885724 Free PMC article.
Cited by
-
Primary care physicians and laypersons' perceptions of multicancer detection clinical trial designs.JNCI Cancer Spectr. 2024 Sep 2;8(5):pkae084. doi: 10.1093/jncics/pkae084. JNCI Cancer Spectr. 2024. PMID: 39270066 Free PMC article.
-
Racial and ethnic minority participants in clinical trials of acute respiratory distress syndrome.Intensive Care Med. 2023 Dec;49(12):1479-1488. doi: 10.1007/s00134-023-07238-x. Epub 2023 Oct 17. Intensive Care Med. 2023. PMID: 37847403 Free PMC article.
-
Eliminating Health Disparities in Atrial Fibrillation, Heart Failure, and Dyslipidemia: A Path Toward Achieving Pharmacoequity.Curr Atheroscler Rep. 2023 Dec;25(12):1113-1127. doi: 10.1007/s11883-023-01180-5. Epub 2023 Dec 18. Curr Atheroscler Rep. 2023. PMID: 38108997 Free PMC article. Review.
-
Temporal Trends of Enrollment by Sex and Race in Major Cardiovascular Randomized Clinical Trials.CJC Open. 2023 Nov 2;6(2Part B):454-462. doi: 10.1016/j.cjco.2023.10.015. eCollection 2024 Feb. CJC Open. 2023. PMID: 38487060 Free PMC article.
-
Development of START-EDI guidelines for reporting equality, diversity and inclusion in research: a study protocol.BMJ Open. 2025 Jul 16;15(7):e095778. doi: 10.1136/bmjopen-2024-095778. BMJ Open. 2025. PMID: 40669893 Free PMC article.
References
-
- Carnethon MR, Pu J, Howard G, et al.; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136 (21):e393–e423. doi:10.1161/CIR.0000000000000534 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

