In vitro culture of ovine embryos up to early gastrulating stages
- PMID: 35319748
- PMCID: PMC8977095
- DOI: 10.1242/dev.199743
In vitro culture of ovine embryos up to early gastrulating stages
Abstract
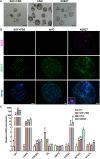
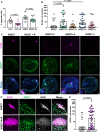
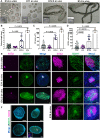
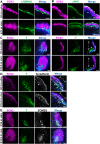
Developmental failures occurring shortly after blastocyst hatching from the zona pellucida constitute a major cause of pregnancy losses in both humans and farm ungulates. The developmental events occurring following hatching in ungulates include the proliferation and maturation of extra-embryonic membranes - trophoblast and hypoblast - and the formation of a flat embryonic disc, similar to that found in humans, which initiates gastrulation prior to implantation. Unfortunately, our understanding of these key processes for embryo survival is limited because current culture systems cannot sustain ungulate embryo development beyond hatching. Here, we report a culture system that recapitulates most developmental landmarks of gastrulating ovine embryos: trophoblast maturation, hypoblast migration, embryonic disc formation, disappearance of the Rauber's layer, epiblast polarization and mesoderm differentiation. Our system represents a highly valuable platform for exploring the cell differentiation, proliferation and migration processes governing gastrulation in a flat embryonic disc and for understanding pregnancy failures during the second week of gestation. This article has an associated 'The people behind the papers' interview.
Keywords: In vitro; Embryo; Gastrulation; Ovine; Post-hatching culture.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Lineage Differentiation Markers as a Proxy for Embryo Viability in Farm Ungulates.Front Vet Sci. 2021 Jun 15;8:680539. doi: 10.3389/fvets.2021.680539. eCollection 2021. Front Vet Sci. 2021. PMID: 34212020 Free PMC article.
-
Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos.Reproduction. 2003 Apr;125(4):607-23. Reproduction. 2003. PMID: 12683931
-
Morphological and Gene Expression Changes in Cattle Embryos from Hatched Blastocyst to Early Gastrulation Stages after Transfer of In Vitro Produced Embryos.PLoS One. 2015 Jun 15;10(6):e0129787. doi: 10.1371/journal.pone.0129787. eCollection 2015. PLoS One. 2015. PMID: 26076128 Free PMC article.
-
Transforming growth factor beta (TGFβ) pathway is essential for hypoblast and epiblast development in ovine post-hatching embryos.Theriogenology. 2023 Jan 15;196:112-120. doi: 10.1016/j.theriogenology.2022.11.021. Epub 2022 Nov 15. Theriogenology. 2023. PMID: 36413867
-
From zygote to implantation: morphological and molecular dynamics during embryo development in the pig.Reprod Domest Anim. 2009 Sep;44 Suppl 3:39-49. doi: 10.1111/j.1439-0531.2009.01482.x. Reprod Domest Anim. 2009. PMID: 19660079 Review.
Cited by
-
Understanding bovine embryo elongation: a transcriptomic study of trophoblastic vesicles.Front Physiol. 2024 Jan 29;15:1331098. doi: 10.3389/fphys.2024.1331098. eCollection 2024. Front Physiol. 2024. PMID: 38348224 Free PMC article.
-
Pig blastocyst-like structure models from embryonic stem cells.Cell Discov. 2024 Jul 2;10(1):72. doi: 10.1038/s41421-024-00693-w. Cell Discov. 2024. PMID: 38956027 Free PMC article.
-
SMC2 ablation impairs bovine embryo development shortly after blastocyst hatching.Reproduction. 2024 Oct 3;168(5):e240211. doi: 10.1530/REP-24-0211. Print 2024 Nov 1. Reproduction. 2024. PMID: 39231091 Free PMC article.
-
Interleukin-6 supplementation improves bovine conceptus elongation and transcriptomic indicators of developmental competence†.Biol Reprod. 2024 Jul 12;111(1):43-53. doi: 10.1093/biolre/ioae045. Biol Reprod. 2024. PMID: 38519105 Free PMC article.
-
A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as "Uteroid" and "Oviductoid".Biology (Basel). 2022 Jun 29;11(7):987. doi: 10.3390/biology11070987. Biology (Basel). 2022. PMID: 36101367 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

