An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium
- PMID: 35319828
- PMCID: PMC9130918
- DOI: 10.1002/advs.202105408
An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium
Abstract
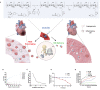
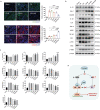
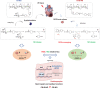
Acute myocardial infarction (MI) is the leading cause of death worldwide. Exogenous delivery of nitric oxide (NO) to the infarcted myocardium has proven to be an effective strategy for treating MI due to the multiple physiological functions of NO. However, reperfusion of blood flow to the ischemic tissues is accompanied by the overproduction of toxic reactive oxygen species (ROS), which can further exacerbate tissue damage and compromise the therapeutic efficacy. Here, an injectable hydrogel is synthesized from the chitosan modified by boronate-protected diazeniumdiolate (CS-B-NO) that can release NO in response to ROS stimulation and thereby modulate ROS/NO disequilibrium after ischemia/reperfusion (I/R) injury. Furthermore, administration of CS-B-NO efficiently attenuated cardiac damage and adverse cardiac remodeling, promoted repair of the heart, and ameliorated cardiac function, unlike a hydrogel that only released NO, in a mouse model of myocardial I/R injury. Mechanistically, regulation of the ROS/NO balance activated the antioxidant defense system and protected against oxidative stress induced by I/R injury via adaptive regulation of the Nrf2-Keap1 pathway. Inflammation is then reduced by inhibition of the activation of NF-κB signaling. Collectively, these results show that this dual-function hydrogel may be a promising candidate for the protection of tissues and organs after I/R injury.
Keywords: inflammation; ischemia/reperfusion injury; nitric oxide; oxidative stress; reactive oxygen species/nitric oxide equilibrium.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mst1 attenuates myocardial ischemia/reperfusion injury following heterotopic heart transplantation in mice through regulating Keap1/Nrf2 axis.Biochem Biophys Res Commun. 2023 Feb 12;644:140-148. doi: 10.1016/j.bbrc.2022.12.087. Epub 2023 Jan 7. Biochem Biophys Res Commun. 2023. PMID: 36646002
-
Keap-NRF2 signaling contributes to the Notch1 protected heart against ischemic reperfusion injury via regulating mitochondrial ROS generation and bioenergetics.Int J Biol Sci. 2022 Feb 7;18(4):1651-1662. doi: 10.7150/ijbs.63297. eCollection 2022. Int J Biol Sci. 2022. PMID: 35280686 Free PMC article.
-
An injectable co-assembled hydrogel blocks reactive oxygen species and inflammation cycle resisting myocardial ischemia-reperfusion injury.Acta Biomater. 2022 Sep 1;149:82-95. doi: 10.1016/j.actbio.2022.06.039. Epub 2022 Jun 29. Acta Biomater. 2022. PMID: 35777549
-
Pharmacological Protection against Ischemia-Reperfusion Injury by Regulating the Nrf2-Keap1-ARE Signaling Pathway.Antioxidants (Basel). 2021 May 21;10(6):823. doi: 10.3390/antiox10060823. Antioxidants (Basel). 2021. PMID: 34063933 Free PMC article. Review.
-
Nrf2 and oxidative stress in liver ischemia/reperfusion injury.FEBS J. 2022 Sep;289(18):5463-5479. doi: 10.1111/febs.16336. Epub 2022 Jan 19. FEBS J. 2022. PMID: 34967991 Review.
Cited by
-
Nanomaterials: Promising Tools for the Diagnosis and Treatment of Myocardial Infarction.Int J Nanomedicine. 2025 Feb 11;20:1747-1768. doi: 10.2147/IJN.S500146. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39958320 Free PMC article. Review.
-
A Powerful Tool in the Treatment of Myocardial Ischemia-Reperfusion Injury: Natural and Nanoscale Modified Small Extracellular Vesicles Derived from Mesenchymal Stem Cells.Int J Nanomedicine. 2023 Dec 28;18:8099-8112. doi: 10.2147/IJN.S443716. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38164265 Free PMC article. Review.
-
Innovative approaches to boost mesenchymal stem cells efficacy in myocardial infarction therapy.Mater Today Bio. 2025 Jan 9;31:101476. doi: 10.1016/j.mtbio.2025.101476. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39896290 Free PMC article. Review.
-
Nanomedicines as Guardians of the Heart: Unleashing the Power of Antioxidants to Alleviate Myocardial Ischemic Injury.Theranostics. 2024 Aug 26;14(13):5336-5370. doi: 10.7150/thno.99961. eCollection 2024. Theranostics. 2024. PMID: 39267789 Free PMC article. Review.
-
Research progress of two-pore potassium channel in myocardial ischemia-reperfusion injury.Front Physiol. 2024 Oct 29;15:1473501. doi: 10.3389/fphys.2024.1473501. eCollection 2024. Front Physiol. 2024. PMID: 39534859 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
