Sequence Conservation Does Not Always Signify a Functional Imperative as Observed in the Nitroreductase Superfamily
- PMID: 35319879
- PMCID: PMC9018611
- DOI: 10.1021/acs.biochem.2c00037
Sequence Conservation Does Not Always Signify a Functional Imperative as Observed in the Nitroreductase Superfamily
Abstract
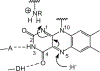
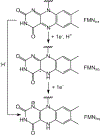
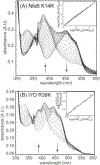
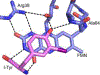
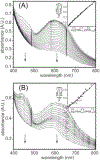
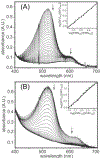
Consensus sequences have the potential to help classify the structure and function of proteins and highlight key regions that may contribute to their biological properties. Often, the level of significance will track with the extent of sequence conservation, but this should not be considered universal. Arg and Lys dominate a position adjacent to the N1 and C2 carbonyl of flavin mononucleotide (FMN) bound in the proteins of the nitroreductase superfamily. Although this placement satisfies expectations for stabilizing the reduced form of FMN, the substitution of these residues in three subfamilies promoting distinct reactions demonstrates their importance to catalysis as only modest. Replacing Arg34 with Lys, Gln, or Glu enhances FMN binding to a flavin destructase (BluB) by twofold and diminishes FMN turnover by no more than 25%. Similarly, replacing Lys14 with Arg, Gln, or Glu in a nitroreductase (NfsB) does not perturb the binding of the substrate nitrofurazone. The catalytic efficiency does decrease by 21-fold for the K14Q variant, but no change in the midpoint potential of FMN was observed with any of the variants. Equivalent substitution at Arg38 in iodotyrosine deiodinase (IYD) affects catalysis even more modestly (<10-fold). While the Arg/Lys to Glu substitution inactivates NfsB and IYD, this change also stabilizes one-electron transfer in IYD contrary to predictions based on other classes of flavoproteins. Accordingly, functional correlations developed in certain structural superfamilies may not necessarily translate well to other superfamilies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

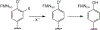


Similar articles
-
A switch between one- and two-electron chemistry of the human flavoprotein iodotyrosine deiodinase is controlled by substrate.J Biol Chem. 2015 Jan 2;290(1):590-600. doi: 10.1074/jbc.M114.605964. Epub 2014 Nov 13. J Biol Chem. 2015. PMID: 25395621 Free PMC article.
-
The 2'-hydroxy group of flavin mononucleotide influences the catalytic function and promiscuity of the flavoprotein iodotyrosine dehalogenase.RSC Chem Biol. 2023 Aug 4;4(9):698-705. doi: 10.1039/d3cb00094j. eCollection 2023 Aug 30. RSC Chem Biol. 2023. PMID: 37654510 Free PMC article.
-
Structural basis for the transformation of the traditional medicine berberine by bacterial nitroreductase.Acta Crystallogr D Struct Biol. 2022 Oct 1;78(Pt 10):1273-1282. doi: 10.1107/S2059798322008373. Epub 2022 Sep 27. Acta Crystallogr D Struct Biol. 2022. PMID: 36189746
-
The FMN-dependent two-component monooxygenase systems.Arch Biochem Biophys. 2010 May;497(1-2):1-12. doi: 10.1016/j.abb.2010.02.007. Epub 2010 Mar 1. Arch Biochem Biophys. 2010. PMID: 20193654 Review.
-
The ins and outs of the flavin mononucleotide cofactor of respiratory complex I.IUBMB Life. 2022 Jul;74(7):629-644. doi: 10.1002/iub.2600. Epub 2022 Feb 14. IUBMB Life. 2022. PMID: 35166025 Review.
Cited by
-
Directed evolution of phosphite dehydrogenase to cycle noncanonical redox cofactors via universal growth selection platform.Nat Commun. 2022 Aug 26;13(1):5021. doi: 10.1038/s41467-022-32727-w. Nat Commun. 2022. PMID: 36028482 Free PMC article.
-
The Catalysis Mechanism of E. coli Nitroreductase A, a Candidate for Gene-Directed Prodrug Therapy: Potentiometric and Substrate Specificity Studies.Int J Mol Sci. 2024 Apr 17;25(8):4413. doi: 10.3390/ijms25084413. Int J Mol Sci. 2024. PMID: 38673999 Free PMC article.
-
Polar Interactions between Substrate and Flavin Control Iodotyrosine Deiodinase Function.Biochemistry. 2024 Sep 17;63(18):2380-2389. doi: 10.1021/acs.biochem.4c00357. Epub 2024 Aug 30. Biochemistry. 2024. PMID: 39213510
References
-
- Copp JN; Babbit PC; Tokuriki N Revealing unexplored sequence-function space using sequence similarity networks. Biochemistry 2018, 57, 4651–4662. - PubMed
-
- Roldán MD; Pérez-Reinado E; Castillo F; Moreno-Vivián C Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol. Rev 2008, 32, 474–500. - PubMed
-
- Race PR; Lovering AL; Green RM; Ossor A; White SA; Searle PF; Wrighton CJ; Hyde EI Structural and mechanistic studies of Escherichia coli nitroreductase with the antibiotic nitrofurazone. Reversed binding orientations in different redox states of the enzyme. J. Biol. Chem 2005, 280, 13256–13264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

