Coreceptor functions of cell surface heparan sulfate proteoglycans
- PMID: 35319900
- PMCID: PMC9109798
- DOI: 10.1152/ajpcell.00050.2022
Coreceptor functions of cell surface heparan sulfate proteoglycans
Abstract
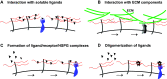
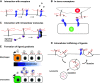
Receptor-ligand interactions play an important role in many biological processes by triggering specific cellular responses. These interactions are frequently regulated by coreceptors that facilitate, alter, or inhibit signaling. Coreceptors work in parallel with other specific and accessory molecules to coordinate receptor-ligand interactions. Cell surface heparan sulfate proteoglycans (HSPGs) function as unique coreceptors because they can bind to many ligands and receptors through their HS and core protein motifs. Cell surface HSPGs are typically expressed in abundance of the signaling receptors and, thus, are capable of mediating the initial binding of ligands to the cell surface. HSPG coreceptors do not possess kinase domains or intrinsic enzyme activities and, for the most part, binding to cell surface HSPGs does not directly stimulate intracellular signaling. Because of these features, cell surface HSPGs primarily function as coreceptors for many receptor-ligand interactions. Given that cell surface HSPGs are widely conserved, they likely serve fundamental functions to preserve basic physiological processes. Indeed, cell surface HSPGs can support specific cellular interactions with growth factors, morphogens, chemokines, extracellular matrix (ECM) components, and microbial pathogens and their secreted virulence factors. Through these interactions, HSPG coreceptors regulate cell adhesion, proliferation, migration, and differentiation, and impact the onset, progression, and outcome of pathophysiological processes, such as development, tissue repair, inflammation, infection, and tumorigenesis. This review seeks to provide an overview of the various mechanisms of how cell surface HSPGs function as coreceptors.
Keywords: coreceptor; heparan sulfate; proteoglycan; receptor-ligand interaction; signaling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
Figures
Similar articles
-
Heparan sulfate proteoglycans in cancer: Pathogenesis and therapeutic potential.Adv Cancer Res. 2023;157:251-291. doi: 10.1016/bs.acr.2022.08.001. Epub 2022 Aug 29. Adv Cancer Res. 2023. PMID: 36725112 Free PMC article.
-
Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin.Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):e116-27. doi: 10.1161/ATVBAHA.111.235184. Epub 2011 Sep 15. Arterioscler Thromb Vasc Biol. 2011. PMID: 21921258
-
Ligand binding to heparan sulfate proteoglycans induces their aggregation and distribution along actin cytoskeleton.Mol Biol Cell. 1996 Nov;7(11):1771-88. doi: 10.1091/mbc.7.11.1771. Mol Biol Cell. 1996. PMID: 8930899 Free PMC article.
-
Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130545. doi: 10.1098/rstb.2013.0545. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349453 Free PMC article. Review.
-
Heparan Sulfate Proteoglycans in Viral Infection and Treatment: A Special Focus on SARS-CoV-2.Int J Mol Sci. 2021 Jun 18;22(12):6574. doi: 10.3390/ijms22126574. Int J Mol Sci. 2021. PMID: 34207476 Free PMC article. Review.
Cited by
-
Extracellular glypican-1 affects tumor progression and prognosis in esophageal cancer.Cancer Med. 2024 Sep;13(18):e70212. doi: 10.1002/cam4.70212. Cancer Med. 2024. PMID: 39300946 Free PMC article.
-
Glycosaminoglycans' Ability to Promote Wound Healing: From Native Living Macromolecules to Artificial Biomaterials.Adv Sci (Weinh). 2024 Mar;11(9):e2305918. doi: 10.1002/advs.202305918. Epub 2023 Dec 10. Adv Sci (Weinh). 2024. PMID: 38072674 Free PMC article. Review.
-
Trastuzumab Decreases the Expression of G1/S Regulators and Syndecan-4 Proteoglycan in Human Rhabdomyosarcoma.Int J Mol Sci. 2025 Feb 27;26(5):2137. doi: 10.3390/ijms26052137. Int J Mol Sci. 2025. PMID: 40076757 Free PMC article.
-
Identification and characterization of a novel heparinase PCHepII from marine bacterium Puteibacter caeruleilacunae.Sci Rep. 2023 Nov 17;13(1):20112. doi: 10.1038/s41598-023-47493-y. Sci Rep. 2023. PMID: 37978313 Free PMC article.
-
The Glycosaminoglycan Side Chains and Modular Core Proteins of Heparan Sulphate Proteoglycans and the Varied Ways They Provide Tissue Protection by Regulating Physiological Processes and Cellular Behaviour.Int J Mol Sci. 2023 Sep 14;24(18):14101. doi: 10.3390/ijms241814101. Int J Mol Sci. 2023. PMID: 37762403 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

