Landscape of KRASG12C, Associated Genomic Alterations, and Interrelation With Immuno-Oncology Biomarkers in KRAS-Mutated Cancers
- PMID: 35319967
- PMCID: PMC8966967
- DOI: 10.1200/PO.21.00245
Landscape of KRASG12C, Associated Genomic Alterations, and Interrelation With Immuno-Oncology Biomarkers in KRAS-Mutated Cancers
Abstract
Purpose: Promising single-agent activity from sotorasib and adagrasib in KRASG12C-mutant tumors has provided clinical evidence of effective KRAS signaling inhibition. However, comprehensive analysis of KRAS-variant prevalence, genomic alterations, and the relationship between KRAS and immuno-oncology biomarkers is lacking.
Materials and methods: Retrospective analysis of deidentified records from 79,004 patients with various cancers who underwent next-generation sequencing was performed. Fisher's exact test evaluated the association between cancer subtypes and KRAS variants. Logistic regression assessed KRASG12C comutations with other oncogenes and the association between KRAS variants and immuno-oncology biomarkers.
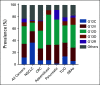
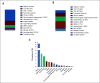
Results: Of the 79,004 samples assessed, 13,758 (17.4%) harbored KRAS mutations, with 1,632 (11.9%) harboring KRASG12C and 12,126 (88.1%) harboring other KRAS variants (KRASnon-G12C). Compared with KRASnon-G12C across all tumor subtypes, KRASG12C was more prevalent in females (56% v 51%, false discovery rate-adjusted P value [FDR-P] = .0006), current or prior smokers (85% v 56%, FDR-P < .0001), and patients age > 60 years (73% v 63%, FDR-P ≤ .0001). The most frequent KRAS variants across all subtypes were G12D (29.5%), G12V (23.0%), G12C (11.9%), G13D (6.5%), and G12R (6.2%). KRASG12C was most prevalent in patients with non-small-cell lung cancer (9%), appendiceal (3.9%), colorectal (3.2%), tumor of unknown origin (1.6%), small bowel (1.43%), and pancreatic (1.3%) cancers. Compared with KRASnon-G12C-mutated, KRASG12C-mutated tumors were significantly associated with tumor mutational burden-high status (17.9% v 8.4%, odds ratio [OR] = 2.38; FDR-P < .0001). KRASG12C-mutated tumors exhibited a distinct comutation profile from KRASnon-G12C-mutated tumors, including higher comutations of STK11 (20.59% v 5.95%, OR = 4.10; FDR-P < .01) and KEAP1 (15.38% v 4.61%, OR = 3.76; FDR-P < .01).
Conclusion: This study presents the first large-scale, pan-cancer genomic characterization of KRASG12C. The KRASG12C mutation was more prevalent in females and older patients and appeared to be associated with smoking status. KRASG12C tumors exhibited a distinct comutation profile and were associated with tumor mutational burden-high status.
Conflict of interest statement
Figures


References
-
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. - PubMed
-
- Martin P, Leighl NB, Tsao MS, et al. KRAS mutations as prognostic and predictive markers in non-small cell lung cancer. J Thorac Oncol. 2013;8:530–542. - PubMed
-
- Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

