Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8
- PMID: 35319989
- PMCID: PMC8942354
- DOI: 10.1126/sciadv.abh4050
Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8
Abstract
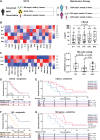
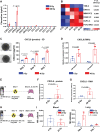
Radiotherapy is a mainstay cancer therapy whose antitumor effects partially depend on T cell responses. However, the role of Natural Killer (NK) cells in radiotherapy remains unclear. Here, using a reverse translational approach, we show a central role of NK cells in the radiation-induced immune response involving a CXCL8/IL-8-dependent mechanism. In a randomized controlled pancreatic cancer trial, CXCL8 increased under radiotherapy, and NK cell positively correlated with prolonged overall survival. Accordingly, NK cells preferentially infiltrated irradiated pancreatic tumors and exhibited CD56dim-like cytotoxic transcriptomic states. In experimental models, NF-κB and mTOR orchestrated radiation-induced CXCL8 secretion from tumor cells with senescence features causing directional migration of CD56dim NK cells, thus linking senescence-associated CXCL8 release to innate immune surveillance of human tumors. Moreover, combined high-dose radiotherapy and adoptive NK cell transfer improved tumor control over monotherapies in xenografted mice, suggesting NK cells combined with radiotherapy as a rational cancer treatment strategy.
Figures





References
-
- Bonner J. A., Harari P. M., Giralt J., Azarnia N., Shin D. M., Cohen R. B., Jones C. U., Sur R., Raben D., Jassem J., Ove R., Kies M. S., Baselga J., Youssoufian H., Amellal N., Rowinsky E. K., Ang K. K., Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006). - PubMed
-
- Klug F., Prakash H., Huber P. E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R. H., Timke C., Umansky L., Klapproth K., Schäkel K., Garbi N., Jäger D., Weitz J., Schmitz-Winnenthal H., Hämmerling G. J., Beckhove P., Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

