Cotranscriptionally encoded RNA strand displacement circuits
- PMID: 35319994
- PMCID: PMC8942360
- DOI: 10.1126/sciadv.abl4354
Cotranscriptionally encoded RNA strand displacement circuits
Abstract
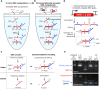
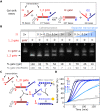
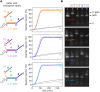
Engineered molecular circuits that process information in biological systems could address emerging human health and biomanufacturing needs. However, such circuits can be difficult to rationally design and scale. DNA-based strand displacement reactions have demonstrated the largest and most computationally powerful molecular circuits to date but are limited in biological systems due to the difficulty in genetically encoding components. Here, we develop scalable cotranscriptionally encoded RNA strand displacement (ctRSD) circuits that are rationally programmed via base pairing interactions. ctRSD circuits address the limitations of DNA-based strand displacement circuits by isothermally producing circuit components via transcription. We demonstrate circuit programmability in vitro by implementing logic and amplification elements, as well as multilayer cascades. Furthermore, we show that circuit kinetics are accurately predicted by a simple model of coupled transcription and strand displacement, enabling model-driven design. We envision ctRSD circuits will enable the rational design of powerful molecular circuits that operate in biological systems, including living cells.
Figures


Similar articles
-
Design Approaches to Expand the Toolkit for Building Cotranscriptionally Encoded RNA Strand Displacement Circuits.ACS Synth Biol. 2023 May 19;12(5):1546-1561. doi: 10.1021/acssynbio.3c00079. Epub 2023 May 3. ACS Synth Biol. 2023. PMID: 37134273
-
Scaling up digital circuit computation with DNA strand displacement cascades.Science. 2011 Jun 3;332(6034):1196-201. doi: 10.1126/science.1200520. Science. 2011. PMID: 21636773
-
Temporal logic circuits implementation using a dual cross-inhibition mechanism based on DNA strand displacement.RSC Adv. 2023 Sep 11;13(39):27125-27134. doi: 10.1039/d3ra03995a. eCollection 2023 Sep 8. RSC Adv. 2023. PMID: 37701285 Free PMC article.
-
Genetically Expressed RNA Strand Displacement for Cellular Manipulation.Chembiochem. 2024 Dec 16;25(24):e202400669. doi: 10.1002/cbic.202400669. Epub 2024 Nov 4. Chembiochem. 2024. PMID: 39304987 Review.
-
DNA Logic Circuits for Cancer Theranostics.Small. 2022 May;18(20):e2108008. doi: 10.1002/smll.202108008. Epub 2022 Mar 7. Small. 2022. PMID: 35254723 Review.
Cited by
-
RNA-Based Sensor Systems for Affordable Diagnostics in the Age of Pandemics.ACS Synth Biol. 2024 Apr 19;13(4):1026-1037. doi: 10.1021/acssynbio.3c00698. Epub 2024 Apr 8. ACS Synth Biol. 2024. PMID: 38588603 Free PMC article. Review.
-
A Study of CRISPR Ribonucleoprotein Displacement in Cell-Free Systems.ACS Omega. 2025 Feb 26;10(9):9154-9164. doi: 10.1021/acsomega.4c09275. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092787 Free PMC article.
-
Dynamic RNA synthetic biology: new principles, practices and potential.RNA Biol. 2023 Jan;20(1):817-829. doi: 10.1080/15476286.2023.2269508. Epub 2023 Dec 3. RNA Biol. 2023. PMID: 38044595 Free PMC article. Review.
-
In vivo-like nearest neighbor parameters improve prediction of fractional RNA base-pairing in cells.Nucleic Acids Res. 2023 Nov 10;51(20):11298-11317. doi: 10.1093/nar/gkad807. Nucleic Acids Res. 2023. PMID: 37855684 Free PMC article.
-
Prevention of ribozyme catalysis through cDNA synthesis enables accurate RT-qPCR measurements of context-dependent ribozyme activity.RNA. 2025 Apr 16;31(5):633-645. doi: 10.1261/rna.080243.124. RNA. 2025. PMID: 40050070
References
-
- Sedlmayer F., Aubel D., Fussenegger M., Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2, 399–415 (2018). - PubMed
-
- Ke J., Wang B., Yoshikuni Y., Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 39, 244–261 (2021). - PubMed
-
- Keasling J. D., Manufacturing molecules through metabolic engineering. Science 330, 1355–1358 (2010). - PubMed
-
- Cherry K. M., Qian L., Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 559, 370–376 (2018). - PubMed
-
- Qian L., Winfree E., Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201 (2011). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

