Opposite effects of stress on effortful motivation in high and low anxiety are mediated by CRHR1 in the VTA
- PMID: 35319997
- PMCID: PMC8942367
- DOI: 10.1126/sciadv.abj9019
Opposite effects of stress on effortful motivation in high and low anxiety are mediated by CRHR1 in the VTA
Abstract
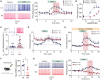
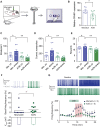
Individuals frequently differ in their behavioral and cognitive responses to stress. However, whether motivation is differently affected by acute stress in different individuals remains to be established. By exploiting natural variation in trait anxiety in outbred Wistar rats, we show that acute stress facilitates effort-related motivation in low anxious animals, while dampening effort in high anxious ones. This model allowed us to address the mechanisms underlying acute stress-induced differences in motivated behavior. We show that CRHR1 expression levels in dopamine neurons of the ventral tegmental area (VTA)-a neuronal type implicated in the regulation of motivation-depend on animals' anxiety, and these differences in CRHR1 expression levels explain the divergent effects of stress on both effortful behavior and the functioning of mesolimbic DA neurons. These findings highlight CRHR1 in VTA DA neurons-whose levels vary with individuals' anxiety-as a switching mechanism determining whether acute stress facilitates or dampens motivation.
Figures




Similar articles
-
Corticosterone Attenuates Reward-Seeking Behavior and Increases Anxiety via D2 Receptor Signaling in Ventral Tegmental Area Dopamine Neurons.J Neurosci. 2021 Feb 17;41(7):1566-1581. doi: 10.1523/JNEUROSCI.2533-20.2020. Epub 2020 Dec 28. J Neurosci. 2021. PMID: 33372063 Free PMC article.
-
Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors.Mol Psychiatry. 2018 Mar;23(3):569-578. doi: 10.1038/mp.2017.135. Epub 2017 Jul 20. Mol Psychiatry. 2018. PMID: 28727688 Free PMC article.
-
Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations.Neuron. 2018 Jan 17;97(2):434-449.e4. doi: 10.1016/j.neuron.2017.12.022. Epub 2018 Jan 4. Neuron. 2018. PMID: 29307710 Free PMC article.
-
Distinct cell populations of ventral tegmental area process motivated behavior.Korean J Physiol Pharmacol. 2022 Sep 1;26(5):307-312. doi: 10.4196/kjpp.2022.26.5.307. Korean J Physiol Pharmacol. 2022. PMID: 36039731 Free PMC article. Review.
-
Lateral hypothalamic area neuropeptides modulate ventral tegmental area dopamine neurons and feeding.Physiol Behav. 2020 Sep 1;223:112986. doi: 10.1016/j.physbeh.2020.112986. Epub 2020 May 31. Physiol Behav. 2020. PMID: 32492498 Free PMC article. Review.
Cited by
-
Hidden variables in stress neurobiology research.Trends Neurosci. 2024 Jan;47(1):9-17. doi: 10.1016/j.tins.2023.10.006. Epub 2023 Nov 18. Trends Neurosci. 2024. PMID: 37985263 Free PMC article. Review.
-
Hypothalamic CRF neurons facilitate brain reward function.Curr Biol. 2024 Jan 22;34(2):389-402.e5. doi: 10.1016/j.cub.2023.12.046. Epub 2024 Jan 11. Curr Biol. 2024. PMID: 38215742 Free PMC article.
-
Transcriptomic analysis reveals mitochondrial pathways associated with distinct adolescent behavioral phenotypes and stress response.Transl Psychiatry. 2023 Nov 17;13(1):351. doi: 10.1038/s41398-023-02648-3. Transl Psychiatry. 2023. PMID: 37978166 Free PMC article.
-
Motivational disturbances in rodent models of neuropsychiatric disorders.Front Behav Neurosci. 2022 Aug 16;16:940672. doi: 10.3389/fnbeh.2022.940672. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36051635 Free PMC article. Review.
-
Intrinsic and extrinsic control impact motivation and outcome sensitivity: the role of anhedonia, stress, and anxiety.Psychol Med. 2024 Dec 27;54(16):1-10. doi: 10.1017/S0033291724002022. Online ahead of print. Psychol Med. 2024. PMID: 39726173 Free PMC article.
References
-
- Korte S. M., Koolhaas J. M., Wingfield J. C., McEwen B. S., The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3–38 (2005). - PubMed
LinkOut - more resources
Full Text Sources

