Subthalamic deep brain stimulation of an anatomically detailed model of the human hyperdirect pathway
- PMID: 35320026
- PMCID: PMC9054256
- DOI: 10.1152/jn.00004.2022
Subthalamic deep brain stimulation of an anatomically detailed model of the human hyperdirect pathway
Abstract
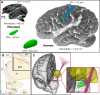
The motor hyperdirect pathway (HDP) is considered a key target in the treatment of Parkinson's disease with subthalamic deep brain stimulation (DBS). This hypothesis is partially derived from the association of HDP activation with evoked potentials (EPs) generated in the motor cortex and subthalamic nucleus (STN) after a DBS pulse. However, the biophysical details of how and when DBS-induced action potentials (APs) in HDP neurons reach their terminations in the cortex or STN remain unclear. Therefore, we used an anatomically detailed representation of the motor HDP, as well as the internal capsule (IC), in a model of human subthalamic DBS to explore AP activation and transmission in the HDP and IC. Our results show that small diameter HDP axons exhibited AP initiation in their subthalamic terminal arbor, which resulted in relatively long transmission latencies to cortex (∼3.5-8 ms). Alternatively, large diameter HDP axons were most likely to be directly activated in the capsular region, which resulted in short transmission times to the cortex (∼1-3 ms). However, those large diameter HDP antidromic APs would be indistinguishable from any other IC axons that were also activated by the stimulus. Conversely, DBS-induced APs in both small and large diameter HDP axons reached their synaptic boutons in the STN with similar timings, but both spanned a wide temporal range (∼0.5-5 ms). We also found that using anodic or bipolar stimulation helped to bias activation of the HDP over the IC. These computational results provide useful information for linking HDP activation with EP recordings in clinical experiments.NEW & NOTEWORTHY We used biophysical models to study pathway recruitment and conduction latencies of the hyperdirect pathway (HDP) in response to subthalamic deep brain stimulation (DBS). The model system allowed us to assess the influence of increased anatomical realism on pathway activity and the possibility of identifying HDP activity in evoked potentials (EPs) recorded in either the subthalamic nucleus (STN) or cortex. The model predicts that HDP activation is accentuated by complex axonal branching in the STN.
Keywords: axon; basal ganglia; deep brain stimulation; motor cortex; subthalamic nucleus.
Conflict of interest statement
C. C. McIntyre is a paid consultant for Boston Scientific Neuromodulation, receives royalties from Hologram Consultants, Neuros Medical, Qr8 Health, and is a shareholder in the following companies: Hologram Consultants, Surgical Information Sciences, CereGate, Autonomic Technologies, Cardionomic, Enspire DBS.
Figures






Similar articles
-
Evolving characterization of the human hyperdirect pathway.Brain Struct Funct. 2023 Mar;228(2):353-365. doi: 10.1007/s00429-023-02610-5. Epub 2023 Jan 28. Brain Struct Funct. 2023. PMID: 36708394 Free PMC article. Review.
-
Model-based deconstruction of cortical evoked potentials generated by subthalamic nucleus deep brain stimulation.J Neurophysiol. 2018 Aug 1;120(2):662-680. doi: 10.1152/jn.00862.2017. Epub 2018 Apr 25. J Neurophysiol. 2018. PMID: 29694280 Free PMC article.
-
Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson's Disease.J Neurosci. 2016 Jan 13;36(2):396-404. doi: 10.1523/JNEUROSCI.2499-15.2016. J Neurosci. 2016. PMID: 26758832 Free PMC article.
-
Cortical Potentials Evoked by Subthalamic Stimulation Demonstrate a Short Latency Hyperdirect Pathway in Humans.J Neurosci. 2018 Oct 24;38(43):9129-9141. doi: 10.1523/JNEUROSCI.1327-18.2018. Epub 2018 Sep 10. J Neurosci. 2018. PMID: 30201770 Free PMC article.
-
Electrophysiological characterization of the hyperdirect pathway and its functional relevance for subthalamic deep brain stimulation.Exp Neurol. 2022 Jun;352:114031. doi: 10.1016/j.expneurol.2022.114031. Epub 2022 Mar 2. Exp Neurol. 2022. PMID: 35247373 Review.
Cited by
-
Prospective Connectomic-Based Deep Brain Stimulation Programming for Parkinson's Disease.Mov Disord. 2024 Dec;39(12):2249-2258. doi: 10.1002/mds.30026. Epub 2024 Oct 21. Mov Disord. 2024. PMID: 39431498
-
Evolving characterization of the human hyperdirect pathway.Brain Struct Funct. 2023 Mar;228(2):353-365. doi: 10.1007/s00429-023-02610-5. Epub 2023 Jan 28. Brain Struct Funct. 2023. PMID: 36708394 Free PMC article. Review.
-
Coupled Activation of the Hyperdirect and Cerebellothalamic Pathways with Zona Incerta Deep Brain Stimulation.Mov Disord. 2024 Mar;39(3):539-545. doi: 10.1002/mds.29717. Epub 2024 Feb 6. Mov Disord. 2024. PMID: 38321526 Free PMC article.
-
Enabling electric field model of microscopically realistic brain.Brain Stimul. 2025 Jan-Feb;18(1):77-93. doi: 10.1016/j.brs.2024.12.1192. Epub 2024 Dec 20. Brain Stimul. 2025. PMID: 39710004
-
DBS-evoked cortical responses index optimal contact orientations and motor outcomes in Parkinson's disease.NPJ Parkinsons Dis. 2023 Mar 11;9(1):37. doi: 10.1038/s41531-023-00474-4. NPJ Parkinsons Dis. 2023. PMID: 36906723 Free PMC article.
References
-
- Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y, Volkmann J, Krauss JK. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15: 148–160, 2019. doi:10.1038/s41582-018-0128-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

