Bursting emerges from the complementary roles of neurons in a four-cell network
- PMID: 35320029
- PMCID: PMC8993528
- DOI: 10.1152/jn.00017.2022
Bursting emerges from the complementary roles of neurons in a four-cell network
Abstract
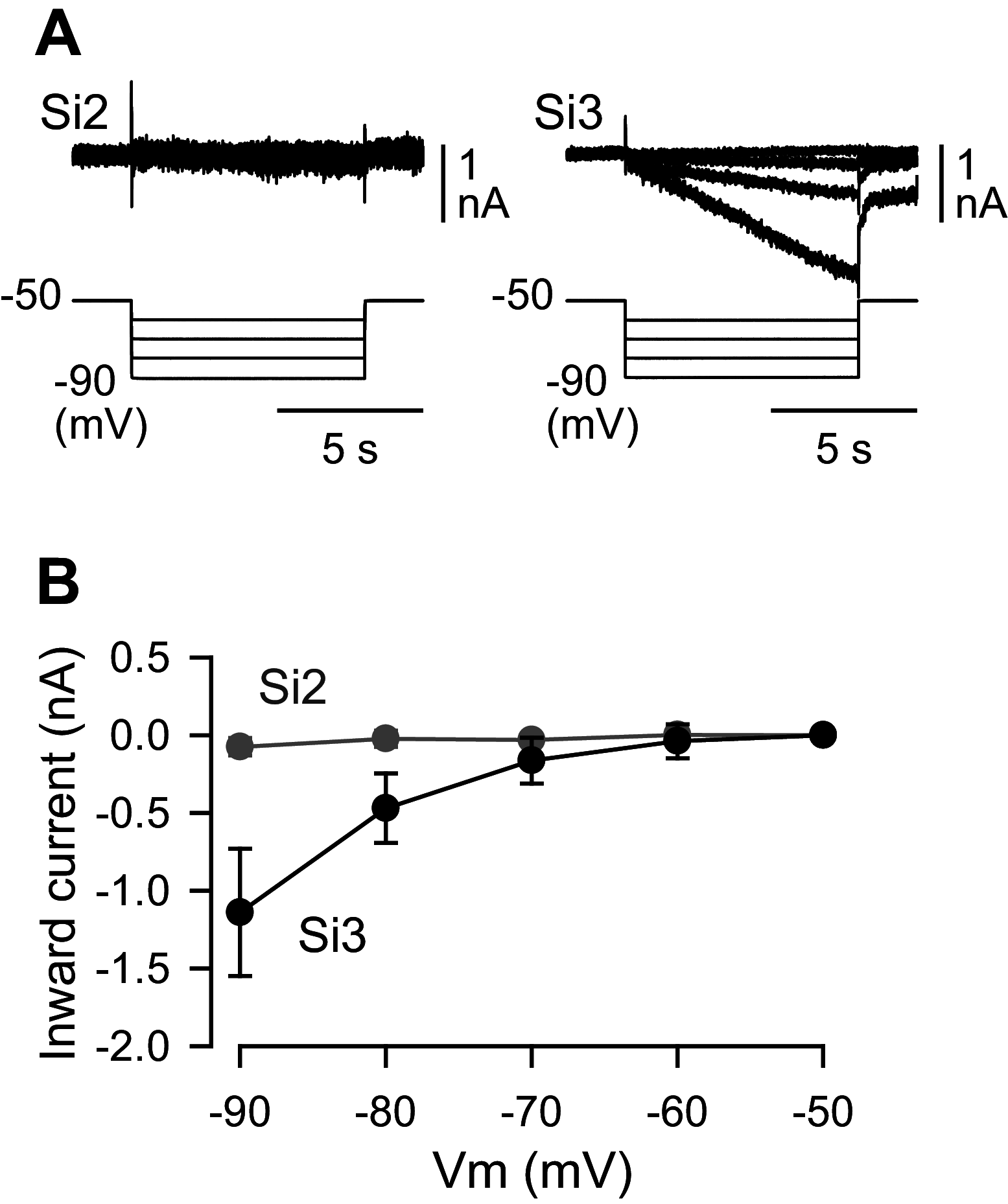
Reciprocally inhibitory modules that form half-center oscillators require mechanisms for escaping or being released from inhibition. The central pattern generator underlying swimming by the nudibranch mollusc, Dendronotus iris, is composed of only four neurons that are organized into two competing modules of a half-center oscillator. In this system, bursting activity in left-right alternation is an emergent property of the network as a whole; none of the neurons produces bursts on its own. We found that the unique synaptic actions and membrane properties of the two neurons in each module (Si2 and the contralateral Si3) play complementary roles in generating stable bursting in this network oscillator. Although Si2 and Si3 each inhibits its contralateral counterpart, Si2 plays a dominant role in evoking fast and strong inhibition of the other module, the termination of which initiates postinhibitory rebound in the Si3 of that module by activating a hyperpolarization-activated inward current. Within each module, the synaptic actions and membrane properties of the two neurons complement each other: Si3 excites Si2, which then feeds back slow inhibition to Si3, terminating the burst. Using dynamic clamp, we showed that the magnitude of the slow inhibition sets the period of the oscillator. Thus, the synaptic actions of Si2 provide the hyperpolarization needed for the other module to rebound stably, whereas the membrane properties of Si3 in each module cause it to rebound first and excite Si2 to maintain the burst until terminated by the slow inhibition from Si2, which releases the other module to become active.NEW & NOTEWORTHY Half-center oscillators composed of reciprocally inhibitory neurons have been posited for over a century to underlie the production of rhythmic movements. The Dendronotus swim central pattern generator may be the simplest such circuit with only two pairs of bilaterally represented neurons. This study completes the description of the mechanism by which this network oscillator functions, showing how stable rhythmic activity arises from the complementary membrane and synaptic properties of the two neurons in the competing modules.
Keywords: gastropod; invertebrate; locomotion; rhythmogenesis; voltage clamp.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
The central pattern generator underlying swimming in Dendronotus iris: a simple half-center network oscillator with a twist.J Neurophysiol. 2016 Oct 1;116(4):1728-1742. doi: 10.1152/jn.00150.2016. Epub 2016 Jul 20. J Neurophysiol. 2016. PMID: 27440239 Free PMC article.
-
Artificial Synaptic Rewiring Demonstrates that Distinct Neural Circuit Configurations Underlie Homologous Behaviors.Curr Biol. 2017 Jun 19;27(12):1721-1734.e3. doi: 10.1016/j.cub.2017.05.016. Epub 2017 Jun 1. Curr Biol. 2017. PMID: 28578931
-
Two interconnected kernels of reciprocally inhibitory interneurons underlie alternating left-right swim motor pattern generation in the mollusk Melibe leonina.J Neurophysiol. 2014 Sep 15;112(6):1317-28. doi: 10.1152/jn.00261.2014. Epub 2014 Jun 11. J Neurophysiol. 2014. PMID: 24920032
-
Lamprey spinal interneurons and their roles in swimming activity.Brain Behav Evol. 1996;48(5):287-96. doi: 10.1159/000113207. Brain Behav Evol. 1996. PMID: 8932869 Review.
-
Homology and homoplasy of swimming behaviors and neural circuits in the Nudipleura (Mollusca, Gastropoda, Opisthobranchia).Proc Natl Acad Sci U S A. 2012 Jun 26;109 Suppl 1(Suppl 1):10669-76. doi: 10.1073/pnas.1201877109. Epub 2012 Jun 20. Proc Natl Acad Sci U S A. 2012. PMID: 22723353 Free PMC article. Review.
Cited by
-
The legacies of A. O. Dennis Willows and Peter A. Getting: neuroscience research using Tritonia.J Neurophysiol. 2025 Jan 1;133(1):34-45. doi: 10.1152/jn.00318.2024. Epub 2024 Nov 29. J Neurophysiol. 2025. PMID: 39611858 Review.
-
Pairing cellular and synaptic dynamics into building blocks of rhythmic neural circuits. A tutorial.Front Netw Physiol. 2024 Jun 25;4:1397151. doi: 10.3389/fnetp.2024.1397151. eCollection 2024. Front Netw Physiol. 2024. PMID: 38983123 Free PMC article.
References
-
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B 84: 308–319, 1911. doi:10.1098/rspb.1911.0077. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

