Review: Emerging Eye-Based Diagnostic Technologies for Traumatic Brain Injury
- PMID: 35320105
- PMCID: PMC9888755
- DOI: 10.1109/RBME.2022.3161352
Review: Emerging Eye-Based Diagnostic Technologies for Traumatic Brain Injury
Abstract
The study of ocular manifestations of neurodegenerative disorders, Oculomics, is a growing field of investigation for early diagnostics, enabling structural and chemical biomarkers to be monitored overtime to predict prognosis. Traumatic brain injury (TBI) triggers a cascade of events harmful to the brain, which can lead to neurodegeneration. TBI, termed the "silent epidemic" is becoming a leading cause of death and disability worldwide. There is currently no effective diagnostic tool for TBI, and yet, early-intervention is known to considerably shorten hospital stays, improve outcomes, fasten neurological recovery and lower mortality rates, highlighting the unmet need for techniques capable of rapid and accurate point-of-care diagnostics, implemented in the earliest stages. This review focuses on the latest advances in the main neuropathophysiological responses and the achievements and shortfalls of TBI diagnostic methods. Validated and emerging TBI-indicative biomarkers are outlined and linked to ocular neuro-disorders. Methods detecting structural and chemical ocular responses to TBI are categorised along with prospective chemical and physical sensing techniques. Particular attention is drawn to the potential of Raman spectroscopy as a non-invasive sensing of neurological molecular signatures in the ocular projections of the brain, laying the platform for the first tangible path towards alternative point-of-care diagnostic technologies for TBI.
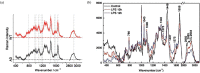
Figures
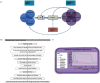

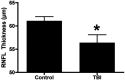
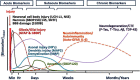
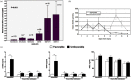



References
-
- Capizzi A., Woo J., and Verduzco-Gutierrez M., “Traumatic brain injury: An overview of epidemiology, pathophysiology, and medical management,” Med. Clin. North Amer., vol. 104, pp. 213–238, 2020. - PubMed
-
- Dewan M. C. et al. , “Estimating the global incidence of traumatic brain injury,” J. Neurosurg., vol. 130, pp. 1080–1097, 2019. - PubMed
-
- Feigin V. L. et al. , “Incidence of traumatic brain injury in New Zealand: A population-based study,” Lancet Neurol., vol. 12, pp. 53–64, 2013. - PubMed
-
- “Traumatic brain injury fact sheets and policy brief,” CENTER-TBI. Accessed: Mar. 31, 2022. [Online]. Available: https://www.center-tbi.eu/files/news/21571f81-20b8-4860-a3dd-1f6e27d02b3...
-
- “Traumatic brain injury and offending, An economic analysis,” Centre for Mental Health. Accessed: Mar. 31, 2022. [Online]. Available: https://www.centreformentalhealth.org.uk/sites/default/files/2018-09/Tra...

