Analysis of transcribed sequences from young and mature zebrafish thrombocytes
- PMID: 35320267
- PMCID: PMC8942222
- DOI: 10.1371/journal.pone.0264776
Analysis of transcribed sequences from young and mature zebrafish thrombocytes
Abstract
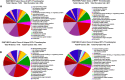
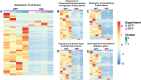
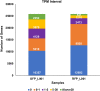
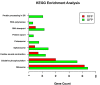
The zebrafish is an excellent model system to study thrombocyte function and development. Due to the difficulties in separating young and mature thrombocytes, comparative transcriptomics between these two cell types has not been performed. It is important to study these differences in order to understand the mechanism of thrombocyte maturation. Here, we performed single-cell RNA sequencing of the young and mature zebrafish thrombocytes and compared the two datasets for young and mature thrombocyte transcripts. We found a total of 9143 genes expressed cumulatively in both young and mature thrombocytes, and among these, 72% of zebrafish thrombocyte-expressed genes have human orthologs according to the Ensembl human genome annotation. We also found 397 uniquely expressed genes in young and 2153 uniquely expressed genes in mature thrombocytes. Of these 397 and 2153 genes, 272 and 1620 corresponded to human orthologous genes, respectively. Of all genes expressed in both young and mature thrombocytes, 4224 have been reported to be expressed in human megakaryocytes, and 1603 were found in platelets. Among these orthologs, 156 transcription factor transcripts in thrombocytes were found in megakaryocytes and 60 transcription factor transcripts were found in platelets including a few already known factors such as Nfe2 and Nfe212a (related to Nfe2) that are present in both megakaryocytes, and platelets. These results indicate that thrombocytes have more megakaryocyte features and since platelets are megakaryocyte fragments, platelets also appear to be thrombocyte equivalents. In conclusion, our study delineates the differential gene expression patterns of young and mature thrombocytes, highlighting the processes regulating thrombocyte maturation. Future knockdown studies of these young and mature thrombocyte-specific genes are feasible and will provide the basis for understanding megakaryocyte maturation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Fallatah W, De Silva IW, Verbeck GF, Jagadeeswaran P. Generation of transgenic zebrafish with 2 populations of RFP- and GFP-labeled thrombocytes: analysis of their lipids. Blood Adv. 2019;3(9):1406–15. Epub 2019/05/06. doi: 10.1182/bloodadvances.2018023960 ; PubMed Central PMCID: PMC6517667. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

