Galectin-1 and -3 in high amounts inhibit angiogenic properties of human retinal microvascular endothelial cells in vitro
- PMID: 35320287
- PMCID: PMC8942239
- DOI: 10.1371/journal.pone.0265805
Galectin-1 and -3 in high amounts inhibit angiogenic properties of human retinal microvascular endothelial cells in vitro
Abstract
Purpose: Galectin-1 and -3 are β-galactoside binding lectins with varying effects on angiogenesis and apoptosis. Since in retinal pigment epithelial cells high amounts of human recombinant galectin (hr-GAL)1 and 3 inhibit cell adhesion, migration and proliferation, we investigated if hr-GAL1 and 3 have homologous effects on human retinal microvascular endothelial cells (HRMEC) in vitro.
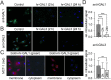
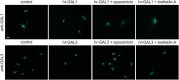
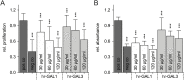
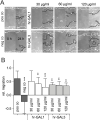
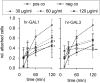
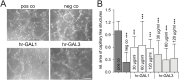
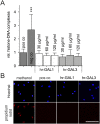
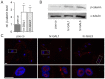
Methods: To investigate the effect of galectin-1 and -3 on HRMEC, proliferation, apoptosis and viability were analyzed after incubation with 30, 60 and 120 μg/ml hr-GAL1 or 3 by BrdU-ELISA, histone-DNA complex ELISA, live/dead staining and the WST-1 assay, respectively. Further on, a cell adhesion as well as tube formation assay were performed on galectin-treated HRMEC. Migration was investigated by the scratch migration assay and time-lapse microscopy. In addition, immunohistochemical staining on HRMEC for β-catenin, galectin-1 and -3 were performed and β-catenin expression was investigated by western blot analysis.
Results: Incubation with hr-GAL1 or 3 lead to a decrease in proliferation, migration, adhesion and tube formation of HRMEC compared to the untreated controls. No toxic effects of hr-GAL1 and 3 on HRMEC were detected. Intriguingly, after treatment of HRMEC with hr-GAL1 or 3, an activation of the proangiogenic Wnt/β-catenin signaling pathway was observed. However, incubation of HRMEC with hr-GAL1 or 3 drew intracellular galectin-1 and -3 out of the cells, respectively.
Conclusion: Exogenously added hr-GAL1 or 3 inhibit angiogenic properties of HRMEC in vitro, an effect that might be mediated via a loss of intracellular endogenous galectins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Galectin-1 Attenuates PDGF-Mediated AKT Signaling in Retinal Pigment Epithelial Cells.Int J Mol Sci. 2024 Aug 27;25(17):9267. doi: 10.3390/ijms25179267. Int J Mol Sci. 2024. PMID: 39273216 Free PMC article.
-
Cathepsin L-induced galectin-1 may act as a proangiogenic factor in the metastasis of high-grade serous carcinoma.J Transl Med. 2019 Jul 3;17(1):216. doi: 10.1186/s12967-019-1963-7. J Transl Med. 2019. PMID: 31269957 Free PMC article.
-
Galectin-1 Controls the Proliferation and Migration of Liver Sinusoidal Endothelial Cells and Their Interaction With Hepatocarcinoma Cells.J Cell Physiol. 2016 Jul;231(7):1522-33. doi: 10.1002/jcp.25244. Epub 2015 Nov 26. J Cell Physiol. 2016. PMID: 26551914
-
Galectin-1 and -9 in angiogenesis: a sweet couple.Glycobiology. 2014 Oct;24(10):915-20. doi: 10.1093/glycob/cwu048. Epub 2014 May 26. Glycobiology. 2014. PMID: 24861051 Review.
-
Galectin-8: a matricellular lectin with key roles in angiogenesis.Glycobiology. 2014 Oct;24(10):907-14. doi: 10.1093/glycob/cwu054. Epub 2014 Jun 16. Glycobiology. 2014. PMID: 24939370 Review.
Cited by
-
Replacement in angiogenesis research: Studying mechanisms of blood vessel development by animal-free in vitro, in vivo and in silico approaches.Front Physiol. 2022 Aug 17;13:981161. doi: 10.3389/fphys.2022.981161. eCollection 2022. Front Physiol. 2022. PMID: 36060683 Free PMC article. Review.
-
Galectin-1 Attenuates PDGF-Mediated AKT Signaling in Retinal Pigment Epithelial Cells.Int J Mol Sci. 2024 Aug 27;25(17):9267. doi: 10.3390/ijms25179267. Int J Mol Sci. 2024. PMID: 39273216 Free PMC article.
-
Endogenous Galectin-1 Modulates Cell Biological Properties of Immortalized Retinal Pigment Epithelial Cells In Vitro.Int J Mol Sci. 2023 Aug 10;24(16):12635. doi: 10.3390/ijms241612635. Int J Mol Sci. 2023. PMID: 37628816 Free PMC article.
-
Down-regulation of histone deacetylase 7 reduces biological activities of retinal microvascular endothelial cells under high glucose condition and related mechanism.Int J Ophthalmol. 2023 Aug 18;16(8):1210-1217. doi: 10.18240/ijo.2023.08.04. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37602334 Free PMC article.
-
Vascular galectins in tumor angiogenesis and cancer immunity.Semin Immunopathol. 2024 Jul 11;46(1-2):3. doi: 10.1007/s00281-024-01014-9. Semin Immunopathol. 2024. PMID: 38990363 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

