Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer
- PMID: 35320348
- PMCID: PMC9167735
- DOI: 10.1158/2159-8290.CD-21-1117
Reverse Transcriptase Inhibition Disrupts Repeat Element Life Cycle in Colorectal Cancer
Abstract
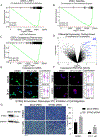
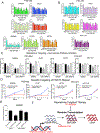
Altered RNA expression of repetitive sequences and retrotransposition are frequently seen in colorectal cancer, implicating a functional importance of repeat activity in cancer progression. We show the nucleoside reverse transcriptase inhibitor 3TC targets activities of these repeat elements in colorectal cancer preclinical models with a preferential effect in p53-mutant cell lines linked with direct binding of p53 to repeat elements. We translate these findings to a human phase II trial of single-agent 3TC treatment in metastatic colorectal cancer with demonstration of clinical benefit in 9 of 32 patients. Analysis of 3TC effects on colorectal cancer tumorspheres demonstrates accumulation of immunogenic RNA:DNA hybrids linked with induction of interferon response genes and DNA damage response. Epigenetic and DNA-damaging agents induce repeat RNAs and have enhanced cytotoxicity with 3TC. These findings identify a vulnerability in colorectal cancer by targeting the viral mimicry of repeat elements.
Significance: Colorectal cancers express abundant repeat elements that have a viral-like life cycle that can be therapeutically targeted with nucleoside reverse transcriptase inhibitors (NRTI) commonly used for viral diseases. NRTIs induce DNA damage and interferon response that provide a new anticancer therapeutic strategy. This article is highlighted in the In This Issue feature, p. 1397.
©2022 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest:
The following authors have filed patents related to targeting repeat RNAs and their use as novel biomarkers (MR, AS, KSA, MNR, VD, BDG, DTT).
DTT and BDG are co-founders, own equity, and receive consulting fees from ROME Therapeutics, a company developing drugs targeting repetitive RNA expressed in cancers and other diseases. This work was not supported by ROME Therapeutics. DTT’s interests were reviewed and are managed by Mass General Brigham in accordance with their conflict of interest policies. Parts of this work was supported by ACD-Biotechne (ASK, VD, MNR, DTT).
DTT has received consulting fees from Tekla Capital Management, Ikena Oncology, NanoString Technologies, Pfizer, Merrimack Pharmaceuticals, Ventana Roche, Foundation Medicine, Inc., and EMD Millipore Sigma, which are not related to this work. DTT is a founder and has equity in PanTher Therapeutics and TellBio, Inc., which are not related to this work.
BDG is a consultant or received honoraria for Darwin Health, Merck, PMV Pharma, ROME Therapeutics, Merck, Bristol–Meyers Squibb, and Chugai Pharmaceuticals and has research funding from Bristol-Meyers Squibb.
ARP is a consultant/advisory board member for Eli Lilly, Natera, Checkmate Pharmaceuticals, Inivata, and Pfizer; holds equity in C2I; serves on the DSMC for Roche; and has research funding from Puretech, PMV Pharmaceuticals, Plexxikon, Takeda, BMS, Novartis, Genentech, Guardant, Array, and Eli Lilly.
MST is a consultant for ROME therapeutics.
JWC is author for McGraw Hill and UpToDate.
LG is a consultant/advisory board member for Alentis, AstraZeneca, Exelixis, and Sirtex, Genentech, Genentech, H3Biomedicine, Incyte, QED Therapeutics, Servier, and Taiho; and has research funding from Adaptimmune, Bayer, Bristol-Myers Squibb, Eisai, Leap Therapeutics, Loxo Oncology, MacroGenics, Merck, Novartis, Nucana, Relay Therapeutics, Genentech, H3Biomedicine, Incyte, QED Therapeutics, Servier, and Taiho.
DPR is a consultant/advisory board member for MPM Capital, Gritstone Oncology, Oncorus, Maverick Therapeutics, 28/7 Therapeutics, Thrive/Exact Sciences; has equity in MPM Capital, Acworth Pharmaceuticals, and Thrive/Exact Sciences; is a legal consultant for Boeringer Ingelheim; and serves as author for Johns Hopkins University Press, UpToDate, McGraw Hill.
RBC is a consultant/advisory board member for Abbvie, Amgen, Array Biopharma/Pfizer, Asana Biosciences, Astex Pharmaceuticals, AstraZeneca, Avidity Biosciences, BMS, C4 Therapeutics, Chugai, Elicio, Erasca, Fog Pharma, Genentech, Guardant Health, Ipsen, Kinnate Biopharma, LOXO, Merrimack, Mirati Therapeutics, Natera, Navire, N-of-one/Qiagen, Novartis, nRichDx, Remix Therapeutics, Revolution Medicines, Roche, Roivant, Shionogi, Shire, Spectrum Pharmaceuticals, Symphogen, Tango Therapeutics, Taiho, Warp Drive Bio, Zikani Therapeutics; holds equity in Avidity Biosciences, C4 Therapeutics, Erasca, Kinnate Biopharma, nRichDx, Remix Therapeutics, and Revolution Medicines; and has research funding from Asana, AstraZeneca, Lilly, Novartis, and Sanofi.
DRW is an inventor of the Simoa technology and a founder, board member, and equity holder of Quanterix Corporation. Interests of DRW have been reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their policies on competing interests.
KHB has consulted for Rome Therapeutics, Tessera Therapeutics, Transposon Therapeutics, and is the Scientific Co-founder of Oncolinea Pharmaceuticals.
All other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

