Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose
- PMID: 35320592
- PMCID: PMC9790192
- DOI: 10.1002/hep.32474
Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose
Erratum in
-
Erratum: Protection and antibody levels 35 years after primary series with hepatitis B vaccine and response to a booster dose.Hepatology. 2024 Nov 1;80(5):E90. doi: 10.1097/HEP.0000000000000982. Epub 2024 Oct 17. Hepatology. 2024. PMID: 39436230 No abstract available.
Abstract
Background and aims: The duration of protection from hepatitis B vaccination in children and adults is not known. In 1981, we used three doses of plasma-derived hepatitis B vaccine to immunize a cohort of 1578 Alaska Native adults and children from 15 Alaska communities who were ≥6 months old.
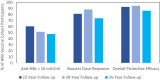
Approach and results: We tested persons for antibody to hepatitis B surface antigen (anti-HBs) levels 35 years after receiving the primary series. Those with levels <10 mIU/ml received one booster dose of recombinant hepatitis B vaccine 2-4 weeks later and were then evaluated on the basis of anti-HBs measurements 30 days postbooster. Among the 320 recruited, 112 persons had not participated in the 22- or 30-year follow-up study (group 1), and 208 persons had participated but were not given an HBV booster dose (group 2). Among the 112 persons in group 1 who responded to the original primary series, 53 (47.3%) had an anti-HBs level ≥10 mIU/ml. Among group 1, 73.7% (28 of 38) of persons available for a booster dose responded to it with an anti-HBs level ≥10 mIU/ml at 30 days. Initial anti-HBs level after the primary series was correlated with higher anti-HBs levels at 35 years. Among 8 persons who tested positive for antibody to hepatitis B core antigen, none tested positive for HBsAg or HBV DNA.
Conclusions: Based on anti-HBs level ≥10 mIU/ml at 35 years and a 73.7% booster dose response, we estimate that 86% of participants had evidence of protection 35 years later. Booster doses are not needed in the general population at this time.
© 2022 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Nothing to report.
Michael G. Bruce: Substantial contribution to conception, design, data acquisition, analysis and interpretation, drafting the article, revision of the article and final approval. Dana Bruden: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Debby Hurlburt: Substantial contribution to data acquisition, analysis, drafting the article. Julie Morris: Substantial contribution to laboratory data acquisition, production and analysis. Sara Bressler: Substantial contribution to data analysis and interpretation, drafting the article, revision of the article. Gail Thompson: Substantial contribution to data acquisition, analysis and interpretation. Danielle Lecy: Substantial contribution to data acquisition, analysis and interpretation. Karen Rudolph: Substantial contribution to laboratory data acquisition, analysis and interpretation. Lisa Bulkow: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Thomas Hennessy: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Brenna C. Simons: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Mark K. Weng: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Noele Nelson: Substantial contribution to data acquisition, analysis and interpretation, drafting the article, and revision of the article. Brian J. McMahon: Substantial contribution to conception, design, data acquisition, analysis and interpretation, drafting the article, revision of the article.
Figures

References
-
- Heyward WL, Bender TR, McMahon BJ, Hall DB, Francis DP, Lanier AP, et al. The control of hepatitis‐B virus‐infection with vaccine in Yupik Eskimos. Demonstration of safety, immunogenicity, and efficacy under field conditions. Am J Epidemiol. 1985;121:914–23. - PubMed
-
- Schreeder MT, Bender TR, McMahon BJ, Moser MR, Murphy BL, Sheller MJ, et al. Prevalence of hepatitis B in selected Alaskan Eskimo villages. Am J Epidemiol. 1983;118:543–9. - PubMed
-
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. - PubMed
-
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15‐year follow‐up. Ann Intern Med. 2005;142:333–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

