T cell depletion increases humoral response by favoring T follicular helper cells expansion
- PMID: 35320600
- PMCID: PMC9262847
- DOI: 10.1111/ajt.17038
T cell depletion increases humoral response by favoring T follicular helper cells expansion
Abstract
Antibody-mediated rejection is a major cause of long-term graft loss in kidney transplant patients. T follicular helper (Tfh) cells are crucial for assisting B cell differentiation and are required for an efficient antibody response. Anti-thymocyte globulin (ATG) is a widely used lymphocyte-depleting induction therapy. However, less is known about how ATG affects Tfh cell development and donor-specific antibody (DSA) formation. We observed an increase in circulating Tfh cells at 6 months after kidney transplant in patients who received ATG. Using an NP-OVA immunization model, we found that ATG-treated mice had a higher percentage of Tfh cells, germinal center B cells, and higher titers of antigen-specific antibodies compared to controls. ATG-treated animals had lower levels of IL-2, a known Bcl-6 repressor, but higher levels of IL-21, pSTAT3 and Bcl-6, favoring Tfh differentiation. In a mouse kidney transplant model, ATG-treated recipients showed an increase in Tfh cells, DSA and C4d staining in the allograft. Although ATG was effective in depleting T cells, it favored the expansion of Tfh cells following depletion. Concomitant use of IL-2, tacrolimus, or rapamycin with ATG was essential to control Tfh cell expansion. In summary, ATG depletion favors Tfh expansion, enhancing antibody-mediated response.
Keywords: DSA; T cell depletion; anti-thymocyte globulin; antibody-mediated rejection; follicular T helper cells; kidney transplantation.
© 2022 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures



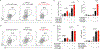


References
-
- Sellarés J et al. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant 12, 388–399 (2012). - PubMed
-
- Cano-Romero FL et al. Longitudinal profile of circulating T follicular helper lymphocytes parallels anti-HLA sensitization in renal transplant recipients. Am. J. Transplant 19, 89–97 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

