Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia
- PMID: 35320643
- PMCID: PMC9107291
- DOI: 10.1056/NEJMoa2114897
Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia
Abstract
Background: Bronchopulmonary dysplasia is a prevalent complication after extremely preterm birth. Inflammation with mechanical ventilation may contribute to its development. Whether hydrocortisone treatment after the second postnatal week can improve survival without bronchopulmonary dysplasia and without adverse neurodevelopmental effects is unknown.
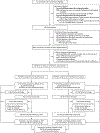
Methods: We conducted a trial involving infants who had a gestational age of less than 30 weeks and who had been intubated for at least 7 days at 14 to 28 days. Infants were randomly assigned to receive either hydrocortisone (4 mg per kilogram of body weight per day tapered over a period of 10 days) or placebo. Mandatory extubation thresholds were specified. The primary efficacy outcome was survival without moderate or severe bronchopulmonary dysplasia at 36 weeks of postmenstrual age, and the primary safety outcome was survival without moderate or severe neurodevelopmental impairment at 22 to 26 months of corrected age.
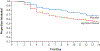
Results: We enrolled 800 infants (mean [±SD] birth weight, 715±167 g; mean gestational age, 24.9±1.5 weeks). Survival without moderate or severe bronchopulmonary dysplasia at 36 weeks occurred in 66 of 398 infants (16.6%) in the hydrocortisone group and in 53 of 402 (13.2%) in the placebo group (adjusted rate ratio, 1.27; 95% confidence interval [CI], 0.93 to 1.74). Two-year outcomes were known for 91.0% of the infants. Survival without moderate or severe neurodevelopmental impairment occurred in 132 of 358 infants (36.9%) in the hydrocortisone group and in 134 of 359 (37.3%) in the placebo group (adjusted rate ratio, 0.98; 95% CI, 0.81 to 1.18). Hypertension that was treated with medication occurred more frequently with hydrocortisone than with placebo (4.3% vs. 1.0%). Other adverse events were similar in the two groups.
Conclusions: In this trial involving preterm infants, hydrocortisone treatment starting on postnatal day 14 to 28 did not result in substantially higher survival without moderate or severe bronchopulmonary dysplasia than placebo. Survival without moderate or severe neurodevelopmental impairment did not differ substantially between the two groups. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT01353313.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Hydrocortisone to Prevent Bronchopulmonary Dysplasia - Not a Silver Bullet.N Engl J Med. 2022 Mar 24;386(12):1181-1183. doi: 10.1056/NEJMe2200247. N Engl J Med. 2022. PMID: 35320649 No abstract available.
-
EBNEO Commentary: Hydrocortisone to improve survival without bronchopulmonary dysplasia.Acta Paediatr. 2022 Nov;111(11):2244-2245. doi: 10.1111/apa.16516. Epub 2022 Aug 29. Acta Paediatr. 2022. PMID: 36036058 No abstract available.
References
-
- Nakashima T, Inoue H, Sakemi Y, Ochiai M, Yamashita H, Ohga S. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J Pediatr 2021; 230: 119–125. e7. - PubMed
-
- Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009; 124: 673–9. - PubMed
-
- Doyle LW, Carse E, Adams A-M, Ranganathan S, Opie G, Cheong JLY. Ventilation in extremely preterm infants and respiratory function at 8 Years. N Engl J Med 2017; 377: 329–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HD021373/HD/NICHD NIH HHS/United States
- UG1 HD087226/HD/NICHD NIH HHS/United States
- U01 HD036790/HD/NICHD NIH HHS/United States
- UL1 TR000442/TR/NCATS NIH HHS/United States
- UL1 TR77/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UL1 TR41/TR/NCATS NIH HHS/United States
- UL1 TR105/TR/NCATS NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
- UL1 TR1117/TR/NCATS NIH HHS/United States
- UG1 HD068263/HD/NICHD NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- UG1 HD068270/HD/NICHD NIH HHS/United States
- UG1 HD068244/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 TR93/TR/NCATS NIH HHS/United States
- UG1 HD040689/HD/NICHD NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR6/TR/NCATS NIH HHS/United States
- UG1 HD027856/HD/NICHD NIH HHS/United States
- UL1 TR42/TR/NCATS NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- UL1 TR454/TR/NCATS NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- UG1 HD068284/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
- UL1 TR442/TR/NCATS NIH HHS/United States
- U24 HD095254/HD/NICHD NIH HHS/United States
