Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa
- PMID: 35320716
- PMCID: PMC9032671
- DOI: 10.1016/j.celrep.2022.110553
Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa
Abstract
The Btla inhibitory receptor limits innate and adaptive immune responses, both preventing the development of autoimmune disease and restraining anti-viral and anti-tumor responses. It remains unclear how the functions of Btla in diverse lymphocytes contribute to immunoregulation. Here, we show that Btla inhibits activation of genes regulating metabolism and cytokine signaling, including Il6 and Hif1a, indicating a regulatory role in humoral immunity. Within mucosal Peyer's patches, we find T-cell-expressed Btla-regulated Tfh cells, while Btla in T or B cells regulates GC B cell numbers. Treg-expressed Btla is required for cell-intrinsic Treg homeostasis that subsequently controls GC B cells. Loss of Btla in lymphocytes results in increased IgA bound to intestinal bacteria, correlating with altered microbial homeostasis and elevations in commensal and pathogenic bacteria. Together our studies provide important insights into how Btla functions as a checkpoint in diverse conventional and regulatory lymphocyte subsets to influence systemic immune responses.
Keywords: B cell; CP: Immunology; CP: Microbiology; IgA; Tfh; Tfr; Treg; autoimmunity; germinal center; inhibitory receptor; microbiome.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

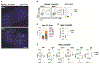





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

