Multi-therapeutic strategy targeting parasite and inflammation-related alterations to improve prognosis of chronic Chagas cardiomyopathy: a hypothesis-based approach
- PMID: 35320825
- PMCID: PMC8944190
- DOI: 10.1590/0074-02760220019
Multi-therapeutic strategy targeting parasite and inflammation-related alterations to improve prognosis of chronic Chagas cardiomyopathy: a hypothesis-based approach
Abstract
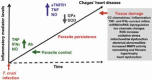
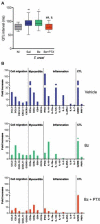
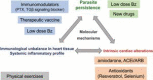
Chagas disease (CD), caused by infection by the protozoan parasite Trypanosoma cruzi, presents as main clinical manifestation the chronic chagasic cardiomyopathy (CCC). CCC afflicts millions of people, mostly in Latin America, and vaccine and effective therapy are still lacking. Comprehension of the host/parasite interplay in the chronic phase of T. cruzi infection may unveil targets for rational trait-based therapies to improve CCC prognosis. In the present viewpoint, I critically summarise a collection of data, obtained by our network of collaborators and other groups on CCC and preclinical studies on pathogenesis, targeting identification for intervention and the use of drugs with immunomodulatory properties to improve CCC. In the last two decades, models combining mouse lineages and T. cruzi strains allowed replication of crucial clinical, histopathological, and immunological traits of CCC. This condition includes conduction changes (heart rate changes, arrhythmias, atrioventricular blocks, prolongation of the QRS complex and PR and corrected QT intervals), ventricular dysfunction and heart failure, CD8-enriched myocarditis, tissue remodeling and progressive fibrosis, and systemic inflammatory profile, resembling "cytokine storm". Studies on Chagas' heart disease pathogenesis begins to unveil the molecular mechanisms underpinning the inflammation-related cardiac tissue damage, placing IFNγ, TNF and NFκB signaling as upstream regulators of miRNAs and mRNAs associated with critical biological pathways as cell migration, inflammation, tissue remodeling and fibrosis, and mitochondrial dysfunction. Further, data on preclinical trials using hypothesis-based tools, targeting parasite and inflammation-related alterations, opened paths for multi-therapeutic approaches in CCC. Despite the long path taken using experimental CD models replicating relevant aspects of CCC and testing new therapies and therapeutic schemes, these findings may get lost in translation, as conceptual and economical challenges, underpinning the valley of death across preclinical and clinical trials. It is hoped that such difficulties will be overcome in the near future.
Figures
Similar articles
-
Cardiac and Digestive Forms of Chagas Disease: An Update on Pathogenesis, Genetics, and Therapeutic Targets.Mediators Inflamm. 2025 Apr 21;2025:8862004. doi: 10.1155/mi/8862004. eCollection 2025. Mediators Inflamm. 2025. PMID: 40297326 Free PMC article. Review.
-
Immunomodulation for the Treatment of Chronic Chagas Disease Cardiomyopathy: A New Approach to an Old Enemy.Front Cell Infect Microbiol. 2021 Nov 12;11:765879. doi: 10.3389/fcimb.2021.765879. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34869068 Free PMC article. Review.
-
In Chagas disease, transforming growth factor beta neutralization reduces Trypanosoma cruzi infection and improves cardiac performance.Front Cell Infect Microbiol. 2022 Nov 30;12:1017040. doi: 10.3389/fcimb.2022.1017040. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36530434 Free PMC article.
-
CCL3/Macrophage Inflammatory Protein-1α Is Dually Involved in Parasite Persistence and Induction of a TNF- and IFNγ-Enriched Inflammatory Milieu in Trypanosoma cruzi-Induced Chronic Cardiomyopathy.Front Immunol. 2020 Mar 3;11:306. doi: 10.3389/fimmu.2020.00306. eCollection 2020. Front Immunol. 2020. PMID: 32194558 Free PMC article.
-
Inflammation and mitochondria in the pathogenesis of chronic Chagas disease cardiomyopathy.Exp Biol Med (Maywood). 2023 Nov;248(22):2062-2071. doi: 10.1177/15353702231220658. Exp Biol Med (Maywood). 2023. PMID: 38235691 Free PMC article. Review.
Cited by
-
Exercise Training Reduces Inflammation and Fibrosis and Preserves Myocardial Function and Perfusion in a Model of Chronic Chagas Cardiomyopathy.Arq Bras Cardiol. 2024 Sep 9;121(8):e20230707. doi: 10.36660/abc.20230707. eCollection 2024. Arq Bras Cardiol. 2024. PMID: 39258653 Free PMC article. English, Portuguese.
-
SBC Guideline on the Diagnosis and Treatment of Patients with Cardiomyopathy of Chagas Disease - 2023.Arq Bras Cardiol. 2023 Jun 26;120(6):e20230269. doi: 10.36660/abc.20230269. Arq Bras Cardiol. 2023. PMID: 37377258 Free PMC article. English, Portuguese. No abstract available.
-
Influence of angiotensin II type 1 receptors and angiotensin-converting enzyme I/D gene polymorphisms on the progression of Chagas' heart disease in a Brazilian cohort: Impact of therapy on clinical outcomes.PLoS Negl Trop Dis. 2024 Nov 26;18(11):e0012703. doi: 10.1371/journal.pntd.0012703. eCollection 2024 Nov. PLoS Negl Trop Dis. 2024. PMID: 39591456 Free PMC article.
-
Cardiac and Digestive Forms of Chagas Disease: An Update on Pathogenesis, Genetics, and Therapeutic Targets.Mediators Inflamm. 2025 Apr 21;2025:8862004. doi: 10.1155/mi/8862004. eCollection 2025. Mediators Inflamm. 2025. PMID: 40297326 Free PMC article. Review.
-
Anxiety, depression, and memory loss in Chagas disease: a puzzle far beyond neuroinflammation to be unpicked and solved.Mem Inst Oswaldo Cruz. 2023 Apr 3;118:e220287. doi: 10.1590/0074-02760220287. eCollection 2023. Mem Inst Oswaldo Cruz. 2023. PMID: 37018799 Free PMC article.
References
-
- Dias JC, Ramos AN, Jr, Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR. et Epidemiol Serv. Saude. 2016;25(spe):7–86. - PubMed
-
- Saraiva RM, Meymandi S. Management of chronic chagasic cardiomyopathy in endemic and non-endemic countries: challenges and limitations. In Delgado MJP, Gascón J, editors. Chagas disease - a neglected tropical disease. Springer. 2020
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

