Ferroptosis as a Novel Determinant of β-Cell Death in Diabetic Conditions
- PMID: 35320979
- PMCID: PMC8938062
- DOI: 10.1155/2022/3873420
Ferroptosis as a Novel Determinant of β-Cell Death in Diabetic Conditions
Abstract
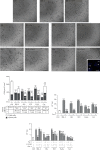
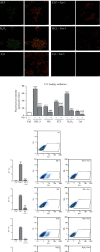
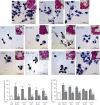
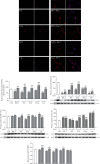
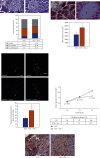
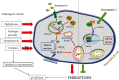
The main pathological hallmark of diabetes is the loss of functional β-cells. Among several types of β-cell death in diabetes, the involvement of ferroptosis remains elusive. Therefore, we investigated the potential of diabetes-mimicking factors: high glucose (HG), proinflammatory cytokines, hydrogen peroxide (H2O2), or diabetogenic agent streptozotocin (STZ) to induce ferroptosis of β-cells in vitro. Furthermore, we tested the contribution of ferroptosis to injury of pancreatic islets in an STZ-induced in vivo diabetic model. All in vitro treatments increased loss of Rin-5F cells along with the accumulation of reactive oxygen species, lipid peroxides and iron, inactivation of NF-E2-related factor 2 (Nrf2), and decrease in glutathione peroxidase 4 expression and mitochondrial membrane potential (MMP). Ferrostatin 1 (Fer-1), ferroptosis inhibitor, diminished the above-stated effects and rescued cells from death in case of HG, STZ, and H2O2 treatments, while failed to increase MMP and to attenuate cell death after the cytokines' treatment. Moreover, Fer-1 protected pancreatic islets from STZ-induced injury in diabetic in vivo model, since it decreased infiltration of macrophages and accumulation of lipid peroxides and increased the population of insulin-positive cells. Such results revealed differences between diabetogenic stimuli in determining the destiny of β-cells, emerging HG, H2O2, and STZ, but not cytokines, as contributing factors to ferroptosis and shed new light on an antidiabetic strategy based on Nrf2 activation. Thus, targeting ferroptosis in diabetes might be a promising new approach for preservation of the β-cell population. Our results obtained from in vivo study strongly justify this approach.
Copyright © 2022 Ana Stancic et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

