Public attitudes to a human challenge study with SARS-CoV-2: a mixed-methods study
- PMID: 35321005
- PMCID: PMC8921687
- DOI: 10.12688/wellcomeopenres.17516.1
Public attitudes to a human challenge study with SARS-CoV-2: a mixed-methods study
Abstract
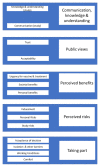
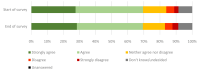
Background: Human challenge studies involve the deliberate exposure of healthy volunteers to an infectious micro-organism in a highly controlled and monitored way. They are used to understand infectious diseases and have contributed to the development of vaccines. In early 2020, the UK started exploring the feasibility of establishing a human challenge study with SARS-CoV-2. Given the significant public interest and the complexity of the potential risks and benefits, it is vital that public views are considered in the design and approval of any such study and that investigators and ethics boards remain accountable to the public. Methods: Mixed methods study comprising online surveys conducted with 2,441 UK adults and in-depth virtual focus groups with 57 UK adults during October 2020 to explore the public's attitudes to a human challenge study with SARS-CoV-2 taking place in the UK. Results: There was overall agreement across the surveys and focus groups that a human challenge study with SARS-CoV-2 should take place in the UK. Transparency of information, trust and the necessity to provide clear information on potential risks to study human challenge study participants were important. The perceived risks of taking part included the risk of developing long-term effects from COVID, impact on personal commitments and mental health implications of isolation. There were a number of practical realities to taking part that would influence a volunteer's ability to participate (e.g. Wi-Fi, access to exercise, outside space and work, family and pet commitments). Conclusions: The results identified practical considerations for teams designing human challenge studies. Recommendations were grouped: 1) messaging to potential study participants, 2) review of the protocol and organisation of the study, and 3) more broadly, making the study more inclusive and relevant. This study highlights the value of public consultation in research, particularly in fields attracting public interest and scrutiny .
Keywords: COVID-19; Ethics; acceptability; consultation; human challenge study; public.
Copyright: © 2022 Barker C et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
-
- NIHR: Over 100,000 volunteers now registered for COVID-19 vaccine trials.2020. Reference Source
-
- Organisation WH: COVID-19 Living map of ongoing research 2020. Reference Source
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

