LncRNA-RMST Functions as a Transcriptional Co-regulator of SOX2 to Regulate miR-1251 in the Progression of Hirschsprung's Disease
- PMID: 35321017
- PMCID: PMC8936393
- DOI: 10.3389/fped.2022.749107
LncRNA-RMST Functions as a Transcriptional Co-regulator of SOX2 to Regulate miR-1251 in the Progression of Hirschsprung's Disease
Abstract
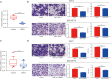
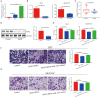
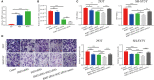
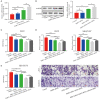
Hirschsprung's disease (HSCR) is a congenital disorder characterized by the absence of enteric neural crest cells (ENCCs). LncRNA rhabdomyosarcoma 2-associated transcript (RMST) is essential for the growth and development of neuron. This study aimed to reveal the role of RMST in the pathogenesis of HSCR. The expression level of RMST, miR-1251, SOX2, and AHNAK was evaluated with qRT-PCR or western blot. CCK-8 and transwell assays were applied to detect cell proliferation and migration. CHIP and RIP assays were applied to determine the combination relationship between SOX2 and promoter region of miR-1251 or RMST and SOX2, respectively. Dual-luciferase reporter assay was performed to confirm miR-1251 targeted AHNAK. As results have shown, RMST was downregulated in the aganglionic colon of HSCR patients. The knockdown of RMST attenuated cell proliferation and migration significantly. MiR-1251, the intronic miRNA of RMST, was also low expressed in HSCR, but RMST did not alter the expression of miR-1251 directly. Furthermore, SOX2 was found to regulate the expression of miR-1251 via binding to the promoter region of miR-1251, and RMST strengthened this function by interacting with SOX2. Moreover, AHNAK was the target gene of miR-1251, which was co-regulated by RMST and SOX2. In conclusion, our study demonstrated that RMST functioned as a transcriptional co-regulator of SOX2 to regulate miR-1251 and resulted in the upregulation of AHNAK, leading to the occurrence of HSCR. The novel RMST/SOX2/miR-1251/AHNAK axis provided potential targets for the diagnosis and treatment of HSCR during embryonic stage.
Keywords: AHNAK; Hirschsprung's disease; SOX2; lncRNA-RMST; miR-1251.
Copyright © 2022 Zhou, Zhi, Chen, Du, Wang, Fang, Tang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Downregulation of microRNA-431-5p promotes enteric neural crest cell proliferation via targeting LRSAM1 in Hirschsprung's disease.Dev Growth Differ. 2019 May;61(4):294-302. doi: 10.1111/dgd.12606. Epub 2019 Apr 29. Dev Growth Differ. 2019. PMID: 31037734
-
LncRNA DRAIC regulates cell proliferation and migration by affecting the miR-34a-5p/ITGA6 signal axis in Hirschsprung's disease.Ups J Med Sci. 2021 Aug 20;126. doi: 10.48101/ujms.v126.7895. eCollection 2021. Ups J Med Sci. 2021. PMID: 34471485 Free PMC article.
-
Downregulation of miR-144 blocked the proliferation and invasion of nerve cells in Hirschsprung disease by regulating Transcription Factor AP 4 (TFAP4).Pediatr Surg Int. 2023 Aug 23;39(1):251. doi: 10.1007/s00383-023-05530-x. Pediatr Surg Int. 2023. PMID: 37610449
-
The roles of non-coding RNAs in Hirschsprung's disease.Noncoding RNA Res. 2024 Feb 28;9(3):704-714. doi: 10.1016/j.ncrna.2024.02.015. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38577013 Free PMC article. Review.
-
RMST: a long noncoding RNA involved in cancer and disease.J Biochem. 2025 Feb 5;177(2):73-78. doi: 10.1093/jb/mvae083. J Biochem. 2025. PMID: 39673327 Review.
Cited by
-
The DNA binding high mobility group box protein family functionally binds RNA.Wiley Interdiscip Rev RNA. 2023 Sep-Oct;14(5):e1778. doi: 10.1002/wrna.1778. Epub 2023 Jan 16. Wiley Interdiscip Rev RNA. 2023. PMID: 36646476 Free PMC article. Review.
-
The lncRNA RMST is drastically downregulated in anaplastic thyroid carcinomas where exerts a tumor suppressor activity impairing epithelial-mesenchymal transition and stemness.Cell Death Discov. 2023 Jul 1;9(1):216. doi: 10.1038/s41420-023-01514-x. Cell Death Discov. 2023. PMID: 37393309 Free PMC article.
-
Identification of Two Long Noncoding RNAs, Kcnq1ot1 and Rmst, as Biomarkers in Chronic Liver Diseases in Mice.Int J Mol Sci. 2024 Aug 16;25(16):8927. doi: 10.3390/ijms25168927. Int J Mol Sci. 2024. PMID: 39201613 Free PMC article.
-
A-to-I-edited miR-1251-5p restrains tumor growth and metastasis in lung adenocarcinoma through regulating TCF7-mediated Wnt signaling pathway.Discov Oncol. 2024 Oct 24;15(1):587. doi: 10.1007/s12672-024-01462-7. Discov Oncol. 2024. PMID: 39446175 Free PMC article.
-
Pathogenic roles of microRNAs and competing endogenous RNAs in Hirschsprung disease (HSCR): Potential diagnostic markers for HSCR.Pediatr Discov. 2023 Jul 31;1(2):e21. doi: 10.1002/pdi3.21. eCollection 2023 Sep. Pediatr Discov. 2023. PMID: 40625718 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

