Dysregulation of Translation in TDP-43 Proteinopathies: Deficits in the RNA Supply Chain and Local Protein Production
- PMID: 35321094
- PMCID: PMC8935057
- DOI: 10.3389/fnins.2022.840357
Dysregulation of Translation in TDP-43 Proteinopathies: Deficits in the RNA Supply Chain and Local Protein Production
Abstract
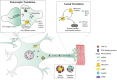
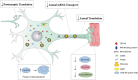
Local control of gene expression provides critical mechanisms for regulating development, maintenance and plasticity in the nervous system. Among the strategies known to govern gene expression locally, mRNA transport and translation have emerged as essential for a neuron's ability to navigate developmental cues, and to establish, strengthen and remove synaptic connections throughout lifespan. Substantiating the role of RNA processing in the nervous system, several RNA binding proteins have been implicated in both developmental and age dependent neurodegenerative disorders. Of these, TDP-43 is an RNA binding protein that has emerged as a common denominator in amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and related disorders due to the identification of causative mutations altering its function and its accumulation in cytoplasmic aggregates observed in a significant fraction of ALS/FTD cases, regardless of etiology. TDP-43 is involved in multiple aspects of RNA processing including splicing, transport and translation. Given that one of the early events in disease pathogenesis is mislocalization from the nucleus to the cytoplasm, several studies have focused on elucidating the pathogenic role of TDP-43 in cytoplasmic translation. Here we review recent findings describing TDP-43 translational targets and potential mechanisms of translation dysregulation in TDP-43 proteinopathies across multiple experimental models including cultured cells, flies, mice and patient derived neurons.
Keywords: ALS; FTD; TDP-43; axon; dendrite; neurodegeneration; synapse; translation.
Copyright © 2022 Bjork, Mortimore, Loganathan and Zarnescu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Altman T., Ionescu A., Ibraheem A., Priesmann D., Gradus-Pery T., Farberov L., et al. (2021). Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 12:6914. 10.1038/s41467-021-27221-8 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

