O6-methylguanine DNA methyltransferase is upregulated in malignant transformation of gastric epithelial cells via its gene promoter DNA hypomethylation
- PMID: 35321285
- PMCID: PMC8919019
- DOI: 10.4251/wjgo.v14.i3.664
O6-methylguanine DNA methyltransferase is upregulated in malignant transformation of gastric epithelial cells via its gene promoter DNA hypomethylation
Abstract
Background: O6-methylguanine-DNA methyltransferase (MGMT) is a suicide enzyme that repairs the mispairing base O6-methyl-guanine induced by environmental and experimental carcinogens. It can transfer the alkyl group to a cysteine residue in its active site and became inactive. The chemical carcinogen N-nitroso compounds (NOCs) can directly bind to the DNA and induce the O6-methylguanine adducts, which is an important cause of gene mutation and tumorigenesis. However, the underlying regulatory mechanism of MGMT involved in NOCs-induced tumorigenesis, especially in the initiation phase, remains largely unclear.
Aim: To investigate the molecular regulatory mechanism of MGMT in NOCs-induced gastric cell malignant transformation and tumorigenesis.
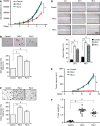
Methods: We established a gastric epithelial cell malignant transformation model induced by N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) or N-methyl-N-nitroso-urea (MNU) treatment. Cell proliferation, colony formation, soft agar, cell migration, and xenograft assays were used to verify the malignant phenotype. By using quantitative real-time polymerase chain reaction (qPCR) and Western blot analysis, we detected the MGMT expression in malignant transformed cells. We also confirmed the MGMT expression in early stage gastric tumor tissues by qPCR and immunohistochemistry. MGMT gene promoter DNA methylation level was analyzed by methylation-specific PCR and bisulfite sequencing PCR. The role of MGMT in cell malignant transformation was analyzed by colony formation and soft agar assays.
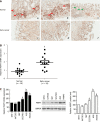
Results: We observed a constant increase in MGMT mRNA and protein expression in gastric epithelial cell malignant transformation induced by MNNG or MNU treatment. Moreover, we found a reduction of MGMT gene promoter methylation level by methylation-specific PCR and bisulfite sequencing PCR in MNNG/MNU-treated cells. Inhibition of the MGMT expression by O6-benzylguanine promoted the MNNG/MNU-induced malignant phenotypes. Overexpression of MGMT partially reversed the cell malignant transformation process induced by MNNG/MNU. Clinical gastric tissue analysis showed that MGMT was upregulated in the precancerous lesions and metaplasia tissues, but downregulated in the gastric cancer tissues.
Conclusion: Our finding indicated that MGMT upregulation is induced via its DNA promoter hypomethylation. The highly expressed MGMT prevents the NOCs-induced cell malignant transformation and tumorigenesis, which suggests a potential novel approach for chemical carcinogenesis intervention by regulating aberrant epigenetic mechanisms.
Keywords: DNA methylation; Epigenetic regulation; Gastric carcinogenesis; Malignant transformation; O6-methylguanine-DNA methyltransferase.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflicts of interest for this article.
Figures



Similar articles
-
Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents.Carcinogenesis. 1991 Oct;12(10):1857-67. doi: 10.1093/carcin/12.10.1857. Carcinogenesis. 1991. PMID: 1657427
-
Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex-, Mex+ and methylation-tolerant mismatch repair compromised cells: facts and models.Mutat Res. 1997 Nov 28;381(2):227-41. doi: 10.1016/s0027-5107(97)00187-5. Mutat Res. 1997. PMID: 9434879
-
Strand- and sequence-specific attenuation of N-methyl-N'-nitro-N-nitrosoguanidine-induced G.C to A.T transitions by expression of human 6-methylguanine-DNA methyltransferase in Chinese hamster ovary cells.Cancer Res. 1994 Jul 15;54(14):3857-63. Cancer Res. 1994. PMID: 8033107
-
O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry.Biochim Biophys Acta. 2011 Dec;1816(2):179-90. doi: 10.1016/j.bbcan.2011.06.002. Epub 2011 Jul 1. Biochim Biophys Acta. 2011. PMID: 21745538 Review.
-
Role of DNA repair in carcinogen-induced ras mutation.Mutat Res. 2000 May 30;450(1-2):139-53. doi: 10.1016/s0027-5107(00)00021-x. Mutat Res. 2000. PMID: 10838139 Review.
Cited by
-
The effect of phytochemicals in N-methyl-N-nitro-N-nitroguanidine promoting the occurrence and development of gastric cancer.Front Pharmacol. 2023 Jun 29;14:1203265. doi: 10.3389/fphar.2023.1203265. eCollection 2023. Front Pharmacol. 2023. PMID: 37456745 Free PMC article. Review.
-
YTHDF1 mediates N-methyl-N-nitrosourea-induced gastric carcinogenesis by controlling HSPH1 translation.Cell Prolif. 2024 Jul;57(7):e13619. doi: 10.1111/cpr.13619. Epub 2024 Mar 5. Cell Prolif. 2024. PMID: 38444279 Free PMC article.
-
Mitochondrial Complex I Molecular Alterations in Sapajus apella as a Human Gastric Carcinogenesis Model After MNU Exposure.J Med Primatol. 2025 Apr;54(2):e70017. doi: 10.1111/jmp.70017. J Med Primatol. 2025. PMID: 40166901 Free PMC article.
-
Microbiota-Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies.Int J Mol Sci. 2024 Jan 30;25(3):1679. doi: 10.3390/ijms25031679. Int J Mol Sci. 2024. PMID: 38338956 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. - PubMed
-
- Padmanabhan N, Ushijima T, Tan P. How to stomach an epigenetic insult: the gastric cancer epigenome. Nat Rev Gastroenterol Hepatol. 2017;14:467–478. - PubMed
-
- Yamashita S, Kishino T, Takahashi T, Shimazu T, Charvat H, Kakugawa Y, Nakajima T, Lee YC, Iida N, Maeda M, Hattori N, Takeshima H, Nagano R, Oda I, Tsugane S, Wu MS, Ushijima T. Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. Proc Natl Acad Sci U S A. 2018;115:1328–1333. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

