Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis
- PMID: 35321742
- PMCID: PMC9491404
- DOI: 10.1186/s12933-022-01476-x
Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis
Abstract
Background: We conducted a systematic review and meta-analysis of the cardiovascular, kidney, and safety outcomes of sodium-glucose cotransporter 2 inhibitors (SGLT2i) among patients with diabetic kidney disease (DKD).
Methods: We searched electronic databases for major randomized placebo-controlled clinical trials published up to September 30, 2021 and reporting on cardiovascular and kidney outcomes of SGLT2i in patients with DKD. DKD was defined as chronic kidney disease in individuals with type 2 diabetes. Random-effects meta-analysis models were used to estimate pooled hazard ratios (HR) and 95% confidence intervals (CI) for clinical outcomes including major adverse cardiovascular events (MACE: myocardial infarction [MI], stroke, and cardiovascular death), kidney composite outcomes (a combination of worsening kidney function, end-stage kidney disease, or death from renal or cardiovascular causes), hospitalizations for heart failure (HHF), deaths and safety events (mycotic infections, diabetic ketoacidosis [DKA], volume depletion, amputations, fractures, urinary tract infections [UTI], acute kidney injury [AKI], and hyperkalemia).
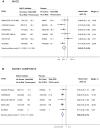
Results: A total of 26,106 participants with DKD from 8 large-scale trials were included (median age: 65.2 years, 29.7-41.8% women, 53.2-93.2% White, median follow-up: 2.5 years). SGLT2i were associated with reduced risks of MACE (HR 0.83, 95% CI 0.75-0.93), kidney composite outcomes (HR 0.66, 95% CI 0.58-0.75), HHF (HR 0.62, 95% CI 0.55-0.71), cardiovascular death (HR 0.84, 95% CI 0.74-0.96), MI (HR 0.78, 95% CI 0.67-0.92), stroke (HR 0.76, 95% CI 0.59-0.97), and all-cause death (HR 0.86, 95% CI 0.77-0.96), with no significant heterogeneity detected. Similar results were observed among participants with reduced estimated glomerular filtration rate (eGFR: < 60 mL/min/1.73m2). The relative risks (95% CI) for adverse events were 3.89 (1.42-10.62) and 2.50 (1.32-4.72) for mycotic infections in men and women respectively, 3.54 (0.82-15.39) for DKA, and 1.29 (1.13-1.48) for volume depletion.
Conclusions: Among adults with DKD, SGLT2i were associated with reduced risks of MACE, kidney outcomes, HHF, and death. With a few exceptions of more clear safety signals, we found overall limited data on the associations between SGLT2i and safety outcomes. More research is needed on the safety profile of SGLT2i in this population.
Keywords: Cardiovascular outcomes; Diabetic kidney disease; Kidney outcomes; Meta-analysis; SGLT2 inhibitors; Safety.
© 2022. The Author(s).
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported. EP is principal investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of the submitted work. SK has received research grants to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche and Bristol-Myers Squibb for studies not related to the topic of the submitted work.
Figures



References
-
- Centers for Disease Control and Prevention, Department of Health and Human Services. National Diabetes statistics report, 2020 estimates of diabetes and its burden in the United States [Internet]. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 7 Oct 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

