Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes
- PMID: 35321874
- PMCID: PMC8943739
- DOI: 10.1136/rmdopen-2021-002074
Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes
Abstract
Introduction: Randomised controlled trials (RCTs) have compared biological and targeted systemic disease-modifying antirheumatic drugs (DMARDS) against placebo in psoriatic arthritis (PsA); few have compared them head to head.
Objectives: To compare the efficacy and safety of all evaluated DMARDs for active PsA, with a special focus on biological DMARDs (bDMARDs) licensed for PsA or psoriasis.
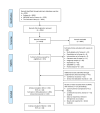
Methods: A systematic review identified RCTs and Bayesian network meta-analysis (NMA) compared treatments on efficacy (American College of Rheumatology (ACR) response, Psoriasis Area and Severity Index (PASI) response, resolution of enthesitis and dactylitis) and safety (patients discontinuing due to adverse events (DAE)) outcomes. Subgroup analyses explored ACR response among patients with and without prior biological therapy exposure.
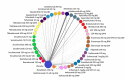
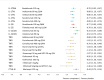
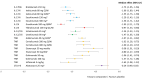
Results: The NMA included 46 studies. Results indicate that some tumour necrosis factor inhibitors (anti-TNFs) may perform numerically, but not significantly, better than interleukin (IL) inhibitors on ACR response but perform worse on PASI response. Few significant differences between bDMARDs on ACR response were observed after subgrouping for prior bDMARD exposure. Guselkumab and IL-17A or IL-17RA inhibitors-brodalumab, ixekizumab, secukinumab-were best on PASI response. These IL-inhibitors and adalimumab were similarly efficacious on resolution of enthesitis and dactylitis. Infliximab with and without methotrexate, certolizumab 400 mg every 4 weeks and tildrakizumab showed the highest rates of DAE; abatacept, golimumab and the IL-inhibitors, the lowest.
Conclusions: Despite similar efficacy for ACR response, IL-17A and IL-17RA inhibitors and guselkumab offered preferential efficacy to anti-TNFs in skin manifestations, and for enthesitis and dactylitis, thereby supporting drug selection based on predominant clinical phenotype.
Keywords: arthritis, psoriatic; biological therapy; outcome assessment, health care.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: IBM has received consulting fees and research funding from Astra Zeneca, Abbvie, Amgen, Boehringer Ingleheim, BMS, Cabaletta, Compugen, Causeway Therapeutics, Eli-Lilly, Evelo, Gilead, Janssen, Novartis, Pfizer, Sanofi, UCB. LMS is an employee of Symmetron Limited and CML was contracted by Symmetron Limited, which received funding from LEO Pharma for this research. CS-G and KM were employed by Symmetron Limited at the time the review was undertaken and the manuscript was written. PH received consulting fees (Eli Lilly) and fees for educational services (Abbvie, Amgen, Pfizer, Novartis, Janssen).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
