Design of stable and self-regulated microbial consortia for chemical synthesis
- PMID: 35322005
- PMCID: PMC8943006
- DOI: 10.1038/s41467-022-29215-6
Design of stable and self-regulated microbial consortia for chemical synthesis
Abstract
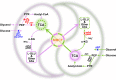
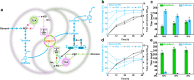
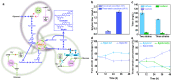
Microbial coculture engineering has emerged as a promising strategy for biomanufacturing. Stability and self-regulation pose a significant challenge for the generation of intrinsically robust cocultures for large-scale applications. Here, we introduce the use of multi-metabolite cross-feeding (MMCF) to establish a close correlation between the strains and the design rules for selecting the appropriate metabolic branches. This leads to an intrinicially stable two-strain coculture where the population composition and the product titer are insensitive to the initial inoculation ratios. With an intermediate-responsive biosensor, the population of the microbial coculture is autonomously balanced to minimize intermediate accumulation. This static-dynamic strategy is extendable to three-strain cocultures, as demonstrated with de novo biosynthesis of silybin/isosilybin. This strategy is generally applicable, paving the way to the industrial application of microbial cocultures.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sun X, et al. Synthesis of chemicals by metabolic engineering of microbes. Chem. Soc. Rev. 2015;44:3760–3785. - PubMed
-
- Biz A, et al. Systems biology based metabolic engineering for non-natural chemicals. Biotechnol. Adv. 2019;37:107379. - PubMed
-
- Lee SY, et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019;2:18–33.
-
- Liu Y, Nielsen J. Recent trends in metabolic engineering of microbial chemical factories. Curr. Opin. Biotechnol. 2019;60:188–197. - PubMed
-
- Sun L, Alper HS. Non-conventional hosts for the production of fuels and chemicals. Curr. Opin. Chem. Biol. 2020;59:15–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

