Lower novelty-related locus coeruleus function is associated with Aβ-related cognitive decline in clinically healthy individuals
- PMID: 35322012
- PMCID: PMC8943159
- DOI: 10.1038/s41467-022-28986-2
Lower novelty-related locus coeruleus function is associated with Aβ-related cognitive decline in clinically healthy individuals
Abstract
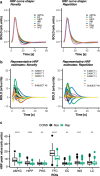
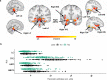
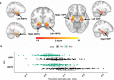
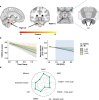
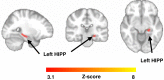
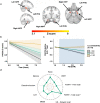
Animal and human imaging research reported that the presence of cortical Alzheimer's Disease's (AD) neuropathology, beta-amyloid and neurofibrillary tau, is associated with altered neuronal activity and circuitry failure, together facilitating clinical progression. The locus coeruleus (LC), one of the initial subcortical regions harboring pretangle hyperphosphorylated tau, has widespread connections to the cortex modulating cognition. Here we investigate whether LC's in-vivo neuronal activity and functional connectivity (FC) are associated with cognitive decline in conjunction with beta-amyloid. We combined functional MRI of a novel versus repeated face-name paradigm, beta-amyloid-PET and longitudinal cognitive data of 128 cognitively unimpaired older individuals. We show that LC activity and LC-FC with amygdala and hippocampus was higher during novelty. We also demonstrated that lower novelty-related LC activity and LC-FC with hippocampus and parahippocampus were associated with steeper beta-amyloid-related cognitive decline. Our results demonstrate the potential of LC's functional properties as a gauge to identify individuals at-risk for AD-related cognitive decline.
© 2022. The Author(s).
Conflict of interest statement
K.V.P. is funded by NIA grant K23 AG053422-01 and the Alzheimer’s Association and has served as a paid consultant for Biogen. A.P.S. has been a paid consultant for Janssen, Biogen, Qynapse, and NervGen. D.M.R. has done consulting for Biogen, Idec and Digital Cognition Technologies and served on the Scientific Advisory Board for Neurotrack. R.F.B is funded by grants from the NIH K99/R00 (R00AG061238) and the Alzheimer’s Association. Y.T.Q is funded by grants from the NIH NIA (R01 AG054671, R01AG066823), the Alzheimer’s Association, and Massachusetts General Hospital ECOR, and has served as a paid consultant for Biogen. K.A.J. has served as paid consultant for Janssen, Genzyme, Novartis, Biogen, Roche, and AC Immune. He is a site co-investigator for Lilly/Avid and Janssen, and receives research support for clinical trials from Eisai, Lilly and Cerveau. K.A.J. received funding from NIH grants R01 EB014894, R21 AG038994, R01 AG026484, R01 AG034556, P50 AG00513421, U19 AG10483, P01 AG036694, R13 AG042201174210, R01 AG027435, and R01 AG037497 and the Alzheimer’s Association grant ZEN-10-174210. RAS has served as a paid consultant for AC Immune, Acumen, Alnylam, Biogen, Cytox, Genentech, Ionis, Janssen, JOMDD, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Roche, Shionogi and receives research support for clinical trials from Alzheimer’s Association, Eisai, Eli Lilly and Co. and NIA. She also receives research support from the following grant: P01 AG036694, U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG0350007, P50 AG005134, U19 AG010483, R01 AG027435, Fidelity Biosciences, Harvard NeuroDiscovery Center and the Alzheimer’s Association. These relationships are not related to the content in the manuscript. All other authors report no relevant conflicts.
Figures

Comment in
-
A novel link between locus coeruleus activity and amyloid-related cognitive decline.Trends Neurosci. 2022 Sep;45(9):651-653. doi: 10.1016/j.tins.2022.05.006. Epub 2022 May 31. Trends Neurosci. 2022. PMID: 35659415 Free PMC article.
Similar articles
-
Association of Novelty-Related Locus Coeruleus Function With Entorhinal Tau Deposition and Memory Decline in Preclinical Alzheimer Disease.Neurology. 2023 Sep 19;101(12):e1206-e1217. doi: 10.1212/WNL.0000000000207646. Epub 2023 Jul 25. Neurology. 2023. PMID: 37491329 Free PMC article.
-
Lower in vivo locus coeruleus integrity is associated with lower cortical thickness in older individuals with elevated Alzheimer's pathology: a cohort study.Alzheimers Res Ther. 2024 Jun 17;16(1):129. doi: 10.1186/s13195-024-01500-0. Alzheimers Res Ther. 2024. PMID: 38886798 Free PMC article.
-
Locus coeruleus integrity and left frontoparietal connectivity provide resilience against attentional decline in preclinical alzheimer's disease.Alzheimers Res Ther. 2024 May 31;16(1):119. doi: 10.1186/s13195-024-01485-w. Alzheimers Res Ther. 2024. PMID: 38822365 Free PMC article.
-
Down but Not Out: The Consequences of Pretangle Tau in the Locus Coeruleus.Neural Plast. 2017;2017:7829507. doi: 10.1155/2017/7829507. Epub 2017 Sep 5. Neural Plast. 2017. PMID: 29038736 Free PMC article. Review.
-
Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer's disease pathogenesis: Potential strategies to protect the LC against aging.Brain Res. 2019 Jan 1;1702:17-28. doi: 10.1016/j.brainres.2017.12.027. Epub 2017 Dec 21. Brain Res. 2019. PMID: 29274876 Review.
Cited by
-
A novel link between locus coeruleus activity and amyloid-related cognitive decline.Trends Neurosci. 2022 Sep;45(9):651-653. doi: 10.1016/j.tins.2022.05.006. Epub 2022 May 31. Trends Neurosci. 2022. PMID: 35659415 Free PMC article.
-
Locus coeruleus activity while awake is associated with REM sleep quality in older individuals.JCI Insight. 2023 Oct 23;8(20):e172008. doi: 10.1172/jci.insight.172008. JCI Insight. 2023. PMID: 37698926 Free PMC article.
-
Social activity mediates locus coeruleus tangle-related cognition in older adults.Mol Psychiatry. 2024 Jul;29(7):2001-2008. doi: 10.1038/s41380-024-02467-y. Epub 2024 Feb 15. Mol Psychiatry. 2024. PMID: 38355788 Free PMC article.
-
REM sleep quality is associated with balanced tonic activity of the locus coeruleus during wakefulness.J Biomed Sci. 2025 Mar 11;32(1):35. doi: 10.1186/s12929-025-01127-9. J Biomed Sci. 2025. PMID: 40069818 Free PMC article.
-
Association of Intrinsic Functional Connectivity between the Locus Coeruleus and Salience Network with Attentional Ability.J Cogn Neurosci. 2023 Oct 1;35(10):1557-1569. doi: 10.1162/jocn_a_02036. J Cogn Neurosci. 2023. PMID: 37584586 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P41 EB022544/EB/NIBIB NIH HHS/United States
- S10 RR021110/RR/NCRR NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- S10 RR019307/RR/NCRR NIH HHS/United States
- S10 OD018035/OD/NIH HHS/United States
- S10 OD010364/OD/NIH HHS/United States
- R01 AG068062/AG/NIA NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- T32 EB013180/EB/NIBIB NIH HHS/United States
- R01 AG046396/AG/NIA NIH HHS/United States
- R01 AG076153/AG/NIA NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- R01 AG062559/AG/NIA NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

